Introduction
HPLC columns are to be consumed by design, but most failures are preventable. By adopting a systematic, step-by-step care and maintenance routine, you can extend your column’s lifespan often to twice its useful life. This not only cuts costs but ensures consistent, robust chromatographic performance critical for product release, method development, and validation work.
But why do columns really fail? Often, it’s not one big mistake but small, avoidable accustomed practices: skipping sample filtration “just once,” rushing mobile phase changes, neglecting guard columns, or leaving columns in aqueous buffers over the weekend.
This guide breaks down practical, field-tested strategies into clear, actionable steps tailored for analysts who already know the basics but want to sharpen their daily practices.
From understanding column wear mechanisms to choosing the right flushing protocols, each section offers insights you can apply immediately in routine work, whether your lab runs hundreds of stability samples or develops new formulations.
By the end, you’ll be equipped to:
- Detect early signs of column degradation.
- Choose mobile phases and buffers wisely.
- Protect your columns with affordable accessories.
- Align your team around best practices and SOPs.
Think of this as your column life insurance policy, backed not by marketing claims but by practical experience from real-world labs.
Understand Why Columns Fail: Root Causes & Early Warning Signs
Before you can effectively protect an HPLC column, you must first understand how it wears out. Most analysts know the big picture: clogging, high backpressure, and loss of efficiency, but fewer track the early indicators and specific mechanisms behind these symptoms.
Here’s what really shortens column life, and what you should watch for daily:
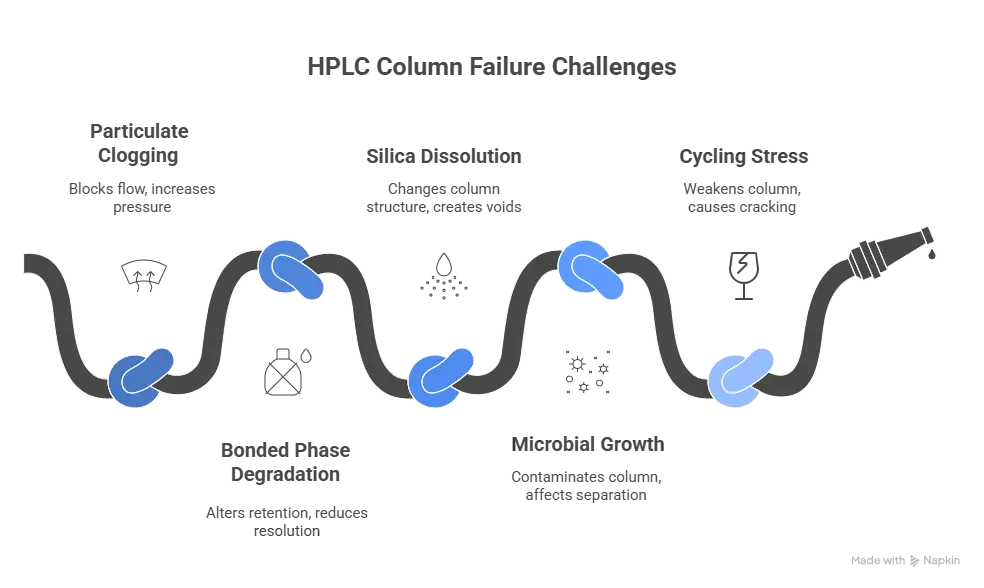
a) Mechanical clogging by particulates
- The number one cause of premature failure.
- Fine particles from samples, buffers, or the environment clog the inlet frit and reduce flow.
- This leads to:
- Gradual increase in backpressure
- Broadening and distortion of early-eluting peaks
- This leads to:
Actionable tip:
– Always filter samples and mobile phases (0.45 µm or finer).
– Consider inline filters or guard columns for high-particulate matrices.
b) Chemical degradation of bonded phase
- Extreme pH, strong acids/bases, or oxidative buffers break down the stationary phase.
- Typical signs:
- Drop in retention for hydrophobic compounds
- Change in selectivity between peaks
Actionable tip:
– Check your method’s pH: stay within the column’s recommended range (usually pH 2–8 for silica-based C18).
– Avoid mixing incompatible solvents abruptly.
c) Chemical dissolution of silica
- Silica starts to dissolve at high pH (>8) or under strong alkaline conditions.
- This shortens life by destroying the column bed, leading to:
- Drop in theoretical plates
- Irreversible pressure drop
Actionable tip:
If you must work near pH 8, choose hybrid silica or polymer-based columns built for higher pH stability.
d) Microbial growth in aqueous mobile phases
- Especially common in buffered water-rich methods left idle.
- Causes sticky build-up, random ghost peaks, and rising backpressure.
Actionable tip:
– Prepare fresh buffers every 1–2 days.
– Flush with organic solvent (like 20–30% ACN) when not running.
e) Thermal and pressure cycling stress
- Repeated large shifts in temperature or sudden pressure changes can crack the packed bed.
- Leads to:
- Loss of resolution
- Irregular peak shape or shoulders
Actionable tip:
– Ramp oven temperature gradually.
– Avoid pressure shocks by starting flow at low rates, then ramping up.
f) Cumulative small mistakes
Often, no single catastrophic event ruins a column; instead, small oversights accumulate:
- Skipping daily flush
- Forgetting guard column replacement
- Running dirty samples late on a Friday without a final flush
Actionable tip:
Build a daily checklist covering mobile phase prep, sample filtration, and end-of-day flush.
Early warning signs you should track!!
| What to watch | What it means |
| Gradual rise in backpressure | Inlet blockage, or particulates |
| Loss of resolution between peaks | Bed damage or stationary phase loss |
| Shifting retention times | pH drift, phase degradation |
| Peak tailing or fronting | phase collapse |
Keep these as part of your column performance log, ideally reviewing weekly.
2) Optimize Mobile Phase Quality & Compatibility
Even the best HPLC column can’t survive poor mobile phase practices. Mobile phase issues silently damage columns from the very first injection and often account for the fastest loss of performance. Here’s how to proactively protect your columns, step by step:
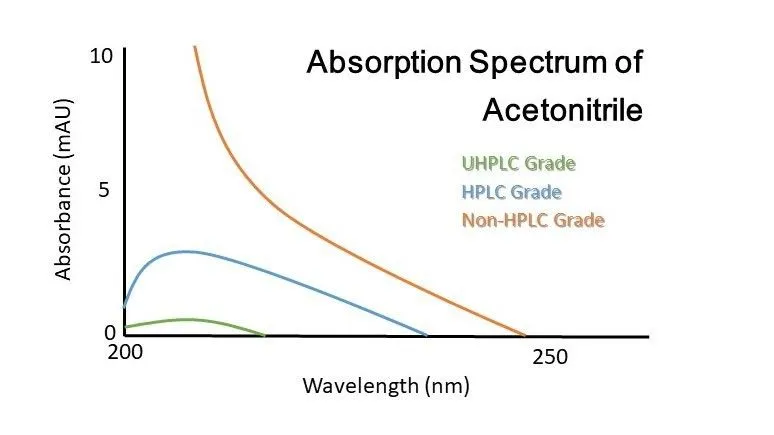
a) Use only high-purity solvents
- Always choose HPLC-grade or LC–MS grade solvents.
- Lower-grade solvents may contain particulate contaminants and UV-active impurities.
- Impurities accumulate on the column, increasing backpressure and causing baseline noise.
Actionable tip:
– Keep dedicated solvent bottles labeled only for HPLC use.
– Never top up partially used bottles; prepare fresh solvent instead.
b) Filter and degas all mobile phases
- Even “clear-looking” mobile phases can have invisible particles.
- Filtration (0.45 µm, ideally 0.2 µm) removes particulates that block frits.
- Degassing removes dissolved gases, preventing bubble formation and baseline drift.
Actionable tip:
– Use in-line degassers if your system has them.
– If not, degas by sonication or helium sparging.
c) Mind your buffer choice and pH
- Buffers stabilize pH but can crystallize, especially phosphate buffers at high organic content.
- Wrong pH damages the stationary phase:
- Low pH (<2): hydrolysis of bonded ligands
- High pH (>8): silica dissolution
Actionable tip:
– Always check column manufacturer’s recommended pH range (commonly ~2–8).
– For critical separations, consider volatile buffers (ammonium acetate/formate) that also suit LC–MS.
d) Watch buffer solubility in organic phasesYour Attractive Heading
- Increasing organic content too quickly can precipitate salts.
- Precipitated buffers clog frits and increase backpressure.
Actionable tip:
– Use gradual gradient programming.
– If storing columns, flush with salt-free solvent first.
e) Prepare fresh mobile phases regularly
- Buffer solutions can support microbial growth, especially in warm labs.
- Bacterial buildup leads to sticky residues and ghost peaks.
Actionable tip:
– Prepare new buffer daily (or every 2 days for low-use systems).
– Add 0.05% sodium azide as a preservative, but confirm compatibility with your detector and application.
f) Control temperature and pressure transitions
- Rapid solvent composition changes can cause phase collapse or bed disruption.
- Sudden temperature changes shift retention times and can crack the packed bed.
Actionable tip:
– After method changes, equilibrate for 10–20 column volumes.
– Increase flow rates gradually at start-up.
g) Document your mobile phase practices
- Keep a mobile phase log noting:
- Batch, preparation date, pH, buffer concentration.
- Observations about clarity, pH stability, or baseline behavior.
This helps trace column performance issues back to mobile phase changes.
Protect with Guard Columns & Inline Filters
Even when your mobile phases and samples are filtered, there’s still risk: trace particulates, matrix residues, or strongly retained compounds can slowly damage your analytical column.
Guard columns and inline filters act like sacrificial shields, absorbing these traces so your main column stays clean and stable longer.
Here’s how to use them effectively:
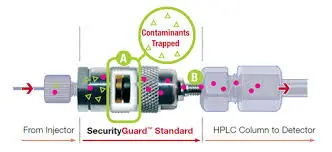
a) Install a compatible guard column
- A short cartridge (often 5–10 mm) packed with the same stationary phase chemistry as your main column (e.g., C18).
- Placed directly upstream of the analytical column.
- Captures particulates and strongly retained impurities before they reach the analytical bed.
Actionable tip:
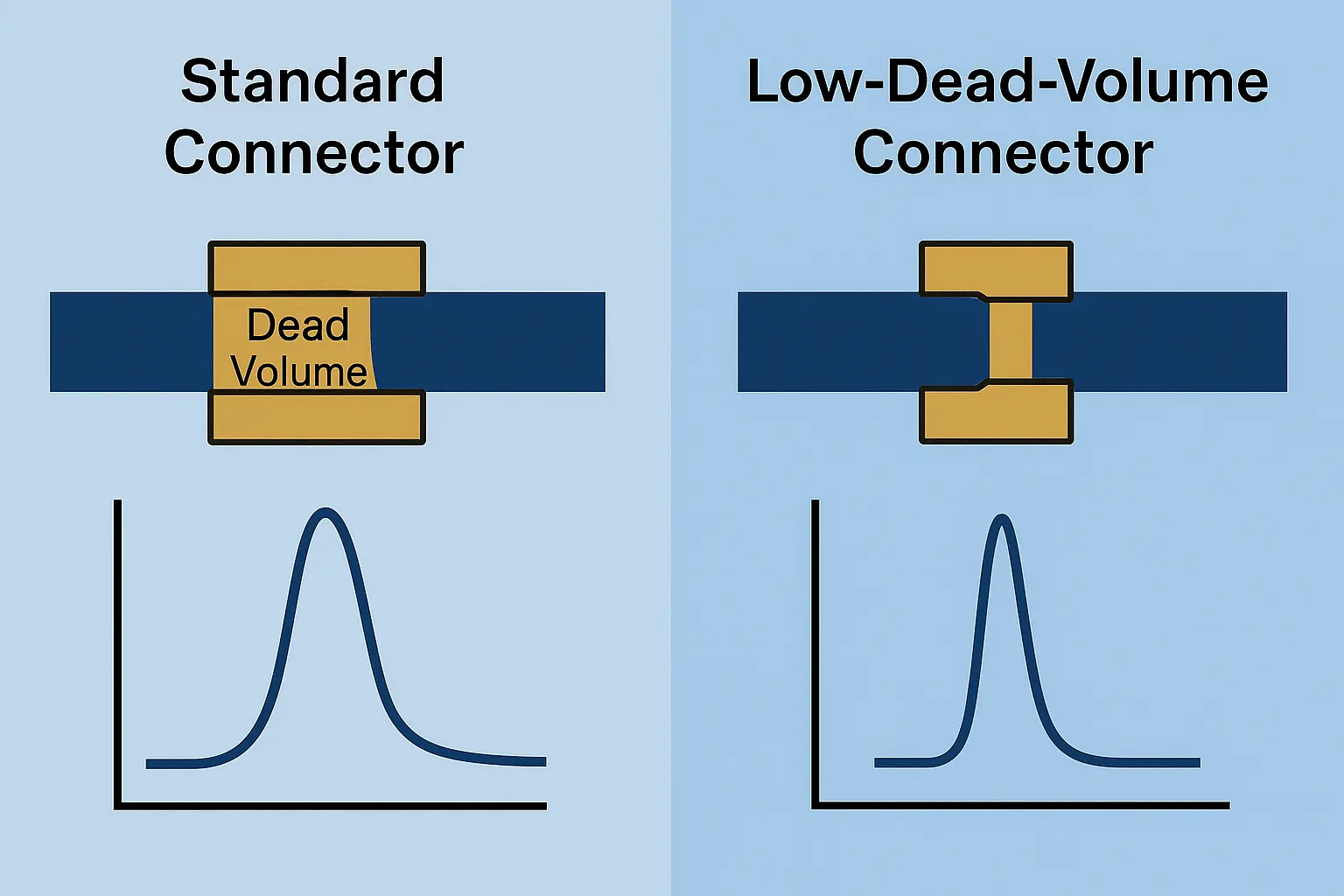
– Match the particle size and phase of the guard to avoid selectivity changes.
– Install using low-dead-volume connectors to maintain peak shape.
b) Add inline filters
- Inline (or pre-column) filters trap non-retained particulates that slip past sample prep.
- Typical pore size: 0.5–2 μm stainless steel frit.
- Especially useful for methods involving:
- Complex biological samples
- Herbal extracts
- High-throughput QC labs

Actionable tip:
– Replace filters proactively, waiting until pressure spikes risks damage to the analytical column.
c) Change guards on a schedule, not just when problems appear
- Waiting for pressure to rise or peaks to degrade is too late.
- Instead, base replacement on:
- Number of injections
- Sample complexity
- Visual signs (color change, deposits)
d) Understand the trade-off: small cost, large savings
- A guard column may cost 5–10% of your main column price.
- But it can double or triple the life of your main column.
- Inline filters cost even less — often under $100 — but protect against catastrophic clogging.
e) Check and document guard column use
- Keep notes on:
- Installation date
- Number of runs
- Observed backpressure
- Tracking this helps optimize replacement frequency and shows auditors your preventive plan.
4) Improve Sample Preparation: Keep the Column Clean from the Start
Your analytical column is only as clean as what you inject into it.
Even the best mobile phase and guard column can’t fully protect against dirty or poorly prepared samples.
Proper sample preparation is often the single most effective step to prevent fouling, reduce backpressure rise, and extend column life.
Here’s how to make sample prep work for you, practically, and without adding unnecessary complexity:
a) Filter every sample before injection
- Use syringe filters (0.45 μm as a minimum; preferably 0.2 μm for LC–MS or small particle columns).
- Even samples that “look clear” often contain invisible particulates.
- These particles accumulate on the inlet frit, causing:
- Rapid backpressure rise
- Poor peak shape
- Risk of clogging during unattended overnight runs
Actionable tip:
– Dedicate filters for each sample type to avoid cross-contamination.
– Train the team to build filtration into every workflow — it becomes routine, not optional.
b) Choose filtration materials carefully
- Membrane type matters:
- Nylon and PVDF: general-purpose
- PTFE: for organic solvents
- PES or regenerated cellulose: for protein-containing or aqueous samples
- CA Cellulose acetate
- GF (Glass fiber)
- Wrong filter material can:
- Leach extractables
- Bind analytes, reducing recovery
Actionable tip:
– Validate your filter choice early during HPLC method development.
c) Use solid phase extraction (SPE) or protein precipitation for complex samples
- Filtration only removes particulates — it doesn’t clean the chemical matrix.
- Biological samples, plant extracts, and stability samples may need:
- Protein precipitation (simple, fast)
- SPE cleanup (more selective)
These steps reduce matrix load, protecting column chemistry from irreversible Column failure.
d) Prepare fresh samples when possible
- Degradation products and microbial growth in stored samples can damage columns.
- If immediate analysis isn’t possible, store samples at controlled temperatures (4 °C or frozen).
e) Control injection volume and solvent strength
- Injecting large volumes or strong solvents can:
- Disturb the stationary phase
- Cause poor peak shape or phase collapse
- Match sample solvent strength to starting mobile phase, or use lower injection volumes.
Actionable tip:
Keep injection volumes consistent; validate the method with your chosen injection size.
f) Document sample prep in method SOPs
- Include:
- Filtration type and pore size
- Dilution steps
- Cleanup techniques
- Standardizing sample prep reduces variability across analysts and shifts.
5) Manage Method Transitions Gently: Protect Your Column from Shocks
Columns often suffer not from normal runs, but from what happens between runs: switching methods, changing mobile phases, or cleaning procedures.
Sudden changes in pH, solvent strength, or temperature can damage the stationary phase, dissolve silica, or crack the packed bed.
Here’s how to transition safely and preserve column life:
a) Change mobile phase composition gradually
- Avoid abrupt solvent changes — especially between high aqueous and high organic content.
- Sudden solvent strength changes can:
- Collapse bonded phases (e.g., C18 chains fall flat in 100% water)
- Precipitate salts from buffers
- Use gradient or stepwise flushing:
- Move from aqueous to nearly 50% organic
- Then to higher organic content as needed
Actionable tip:
Create a short “transition method” in your HPLC software to automate gradual changes.
b) Avoid extreme pH swings
- Large pH jumps can:
- Strip bonded ligands from the stationary phase
- Rapidly dissolve silica at high pH (>8)
- If switching from acidic to basic mobile phases:
- Flush with neutral solvent (e.g., methanol/water) first
Actionable tip:
Always check the pH limits in the column certificate, and share them with your team or preferably write them on the case.
c) Control temperature ramping
- Heating or cooling too fast can crack the packed bed.
- Typical maximum ramp: nearly 5 °C/min.
Actionable tip:
Program oven ramping in the HPLC system, or wait a few minutes after manual temperature change before starting runs.
d) Equilibrate fully after method changes
- After changing mobile phase or gradient profile:
- Flush at least 10–20 column volumes (depending on method complexity).
- Insufficient equilibration leads to:
- Retention time shifts
- Ghost peaks
- Poor reproducibility
Actionable tip:
Add automated equilibration steps to your sequence instead of relying on manual timing.
e) Avoid starting/stopping flow abruptly
- Pumping solvent into a dry column or stopping flow suddenly under pressure can:
- Compact or disrupt the packed bed
- Instead:
- Start flow at a low flow rate (e.g. 0.2 mL/min)
- Increase gradually to method flow rate
Actionable tip:
Create a “start-up method” that automatically ramps flow over 3–5 minutes.
f) Document and standardize your transitions
- Include:
- Flush volumes
- Ramp rates
- Equilibration times
- Helps ensure consistency across analysts and shifts.
Columns don’t wear out most while you’re running them, they often suffer damage when idle, stored improperly, or flushed incorrectly.
Daily, weekly, and long-term flushing and storage habits can make the difference between replacing a column every few months and keeping it productive for a year or more.
Here’s how to do it step by step:
g) Flush at the end of each batch or shift
- Remove retained compounds, salts, and buffer residues.
- Typical procedure:
- Flush with initial mobile phase (5–10 column volumes)
- Switch to high organic (e.g., 90–100% ACN or MeOH)
- Continue until baseline stabilizes and backpressure drops
Actionable tip:
Add an automated “shutdown method” to your sequence, analysts don’t have to remember manually.
h) Match flush solvent to your method
- For non-polar analytes: finish with high organic.
- For ion-pair reagents: consider longer flushes with 100% organic, sometimes mixed with isopropanol.
- For protein-based samples: finish with water–organic–isopropanol mix.
Actionable tip:
Always check column documentation for solvent compatibility.
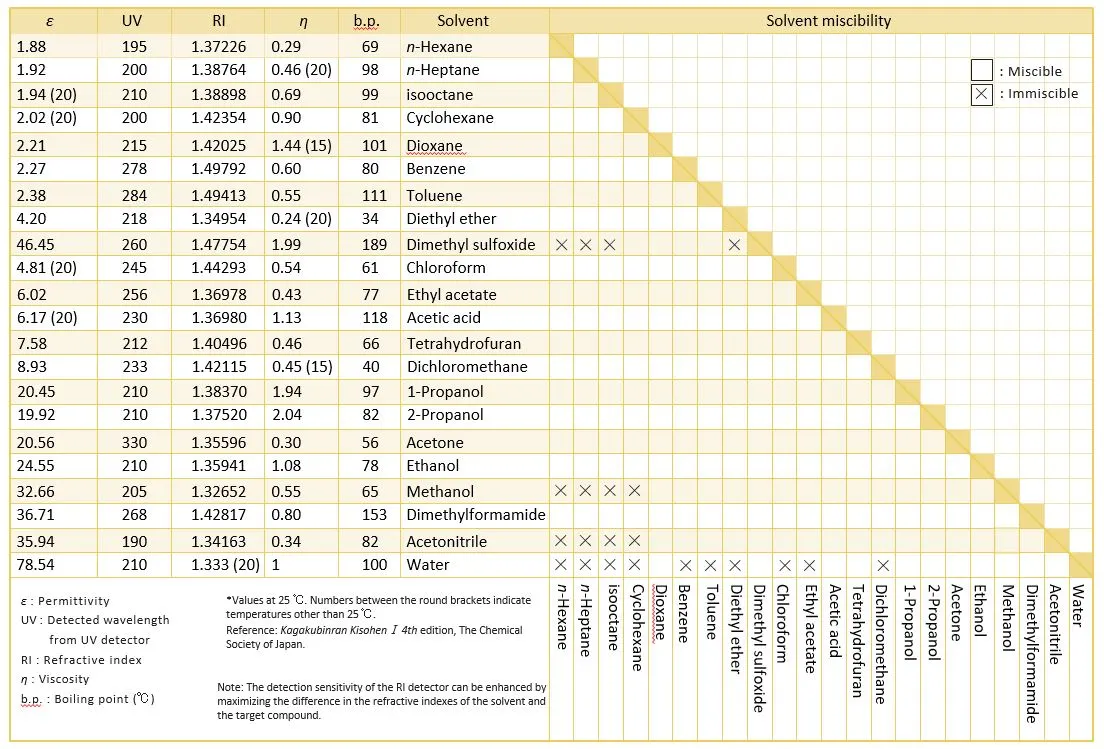
ii) Avoid leaving columns in purely aqueous mobile phases
- Pure water or buffered water promotes:
- Microbial growth
- Phase collapse (for some RP columns)
Actionable tip:
Store in at least 50% organic (e.g., 50:50 ACN:water).
j) Long-term storage (>1 week)
- Flush thoroughly with organic solvent.
- Seal both ends tightly with end plugs or caps.
- Label column tag with:
- Storage solvent
- Last use date
- Analyst initials
Actionable tip:
Keep columns stored vertically (outlet down) to prevent bubble formation.
k) Protect from temperature extremes & light
- Avoid storing columns near heat sources or in sunlight.
- High temperatures accelerate bonded phase degradation.
- Light-sensitive stationary phases can slowly degrade.
ll) Log flushing and storage activities
- Use a simple column log
- Date flushed
- Solvent used
- Storage solvent
- Observed pressure or performance notes
m) Clean aggressively only when needed
- Strong acid, base, or specialized cleaning solutions should be last resort.
- Follow manufacturer’s cleaning guide exactly.
- Improper aggressive cleaning can destroy the bonded phase irreversibly.
7) Monitor Performance Systematically: Detect Problems Before It’s Too Late
Even the best preventive care can’t completely stop wear, but systematic monitoring helps you spot early warning signs, act before columns fail mid-study, and justify replacement when truly needed.
Think of it as building your own real-world performance database.
Here’s how to do it effectively in a QC or R&D environment:
Track key performance indicators (KPIs) regularly
- Backpressure (psi/bar)
- Retention time for standard compound
- Theoretical plates (N)
- Peak symmetry or tailing factor
- Resolution between critical peaks
Actionable tip:
Record after every batch (for high-throughput QC) or weekly (for lower-volume R&D).
Compare against historical baselines
- Use initial validation data or first runs as reference.
- Look for:
- Gradual rise in backpressure
- Shifting retention times
- Decreasing plate count
- Worsening tailing or resolution
Set alert thresholds
Example:
- 20% increase in backpressure → inspect and flush
- Retention time shift >2% → check mobile phase pH and column health
- Helps analysts react early, rather than after failed runs.
Document corrective actions
- Each time you clean, replace guard columns, or change mobile phase
- Log what was done and effect on performance.
- Builds institutional knowledge over time.
Apply data to replacement decisions
- Use trend charts to decide when a column truly needs replacement.
- Helps justify costs and plan procurement proactively.
8) Train and Align Your Team: Make Best Practices Standard Practice
Even the best SOPs and maintenance plans fail if only one person follows them.
To truly protect your HPLC columns and keep data quality high, the entire team QC, R&D, day and night shifts — needs to be aligned and consistent.
Here’s how to make it practical and sustainable:
Build SOPs that are clear, visual, and actionable
- Include:
- Daily flushing routines
- Guard column replacement frequency
- Sample filtration steps
- Mobile phase prep and pH checks
- Use diagrams or quick-reference sheets for complex transitions or cleaning steps.
Actionable tip:
Place SOPs near each HPLC system, not just in shared drives.
Run short, focused training sessions
- Quarterly 20–30 minute refreshers:
- Troubleshooting rising backpressure
- Proper flushing technique
- Recognizing early warning signs
- Interactive discussions: ask analysts to share real column issues they faced.
Share why, not just what
- Explain:
- Why guard columns save money
- Why sudden pH changes cause damage
- Why even filtered buffers can grow microbes
- Understanding root causes makes procedures meaningful, not just checkboxes.
Align across QC and R&D
- Different teams sometimes have different habits.
- Agree on:
- Standard guard column types
- Mobile phase grade
- Flush volumes and solvents
Actionable tip:
Cross-functional meetings (even short) keep everyone consistent.
Make training part of onboarding
- New analysts learn from day one:
- How to flush and store
- How to track performance
- How to handle method transitions
Conclusion
Extending the life of your HPLC columns isn’t about a single “secret trick” it’s about a series of consistent, science-based habits: clean mobile phases, proper flushing, gentle method transitions, guard columns, and systematic monitoring.
By understanding why columns fail and making small daily choices from filtering every sample to logging backpressure trends you turn column care from routine maintenance into a strategic advantage. The payoff isn’t just fewer replacements: it’s greater data integrity, more reliable methods, and smoother lab operations across QC and R&D.
Columns are consumables, but your expertise and discipline decide how consumable they really are.
Invest time upfront, share best practices across your team, and watch your column life and confidence in your results grow.




