Introduction
High-Performance Liquid Chromatography (HPLC) is one of the most versatile and widely used analytical techniques in pharmaceutical, environmental, and chemical analysis. Like any complex analytical method, HPLC systems may encounter problems that hinder performance, accuracy, and reliability despite its robust nature. Troubleshooting HPLC can be challenging, as the causes of issues often stem from various sources such as the mobile phase, sample preparation, system maintenance, and instrument setup. This article delves into common HPLC problems, their root causes, and solutions, backed by highly cited literature sources.
Common HPLC problems include:
1) Inconsistent or Low Resolution:
Resolution refers to the ability of the system to separate analytes effectively. Low resolution may result in peak overlap, making it difficult to quantify and identify different analytes.
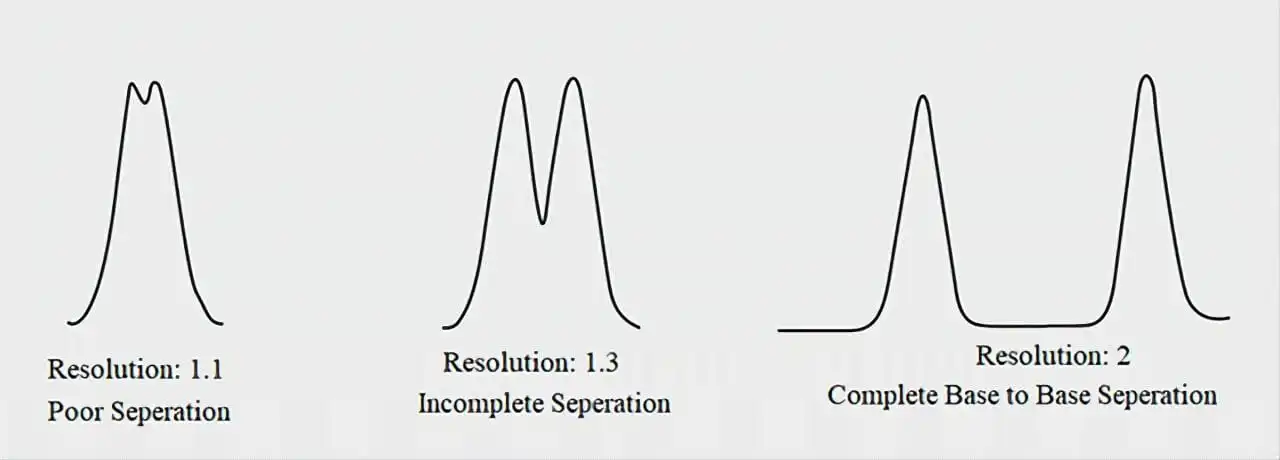
Causes:
- Incorrect mobile phase composition: An improper pH (Especially for structurally similar compounds), ionic strength, or organic modifier concentration can lead to poor separations.
- Column degradation: Columns with clogged or deteriorated packing can cause uneven flow, which leads to broad peaks.
- Excessive sample load: Overloading the column with too much sample can lead to peak tailing, broad peak shape, and low resolution. This is especially problematic with complex samples.
Solutions:
- Mobile phase optimization: Ensure that the pH and ionic strength are within the optimal range for the separation. Adjusting the mobile phase composition or using gradient elution can often improve resolution (by starting with low organic percentage that increases gradually by time allowing for the separation of structurally similar compounds).
- Column maintenance (replacement or regeneration): Regular column cleaning and proper storage can extend column life. In cases of column degradation, regenerate the column using appropriate solvents or replace it. Consider using a guard column to protect the analytical column if possible.
- Sample concentration: Reduce sample concentrations to prevent overloading the column or consider lowering injection volume which will also reduce the solvent effect (discussed later on) affecting separation efficiency.
2) Noisy Baseline or Drift:
A noisy or drifting baseline is often a significant issue in HPLC, particularly when dealing with low-level quantification or complex mixtures. Baseline noise can obscure small peaks, while baseline drift can interfere with the reproducibility of the chromatographic results.
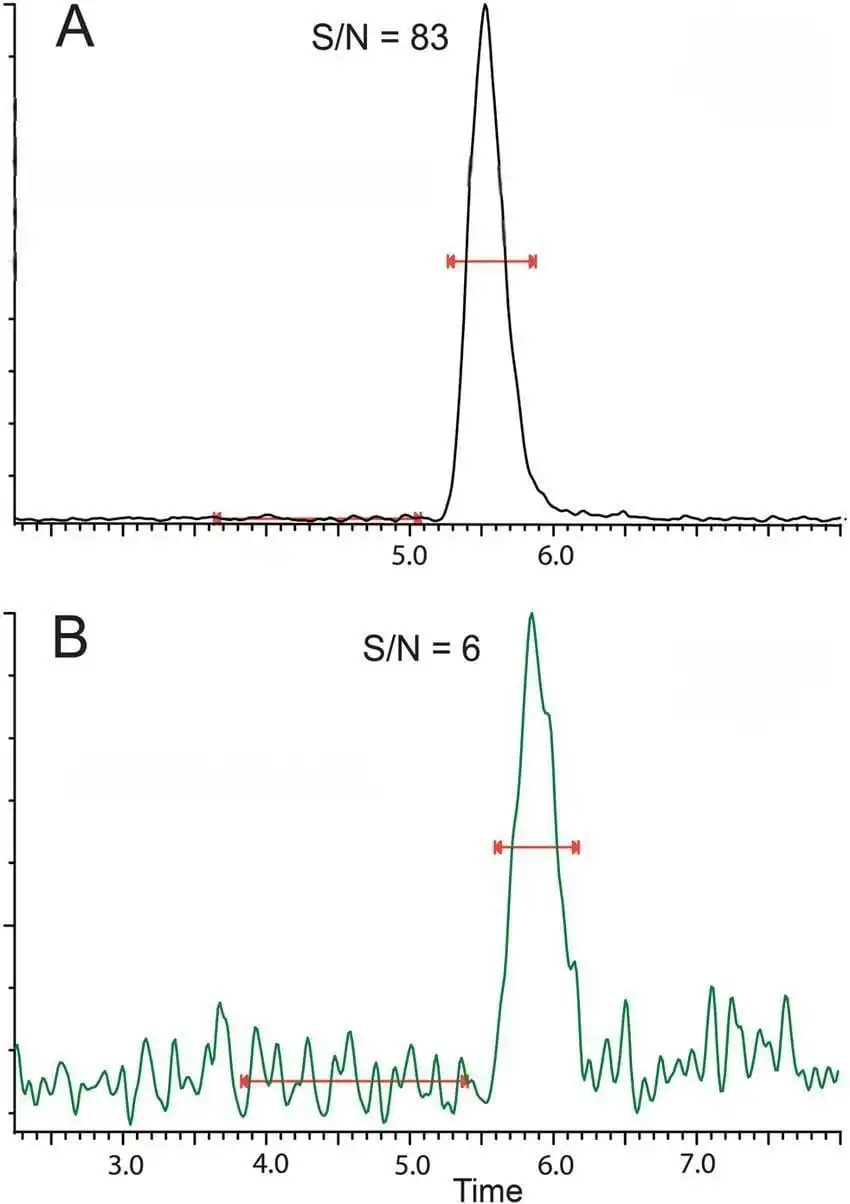
Causes:
- Contamination in Mobile Phase: Impurities in the solvents or air bubbles (inadequate degassing) can result in noisy baselines.
- Detector Instability: Fluctuations in the light source or detector electronics can lead to baseline drift.
- Poor Systemic Sealing: Leaks in the pump, injector, or detector can cause pressure instability, leading to baseline noise.
- Inconsistent Solvent Composition: Solvent changes during the chromatographic run can cause the detector to show inconsistent responses.
- Presence of organic modifiers with high cut-off wavelength: methanol, triethylamine or high molarity buffer solutions can lead to noisy baseline
Solutions:
- Ensure Purity of Solvents: Use high purity solvents and ensure that they are properly filtered and free from particulates that could cause noise. Regularly change solvents to ensure absence of any contamination.
- Degassing the mobile phase: Use a degassing system or sonicate the mobile phase to remove dissolved gases.
- Regular Detector Maintenance: Periodically calibrate and check for issues with the light source, optical path, and detector electronics. Ensure that the detector is aligned and free from contaminants.
- Leak Prevention: Regularly inspect the system for leaks, particularly around seals, connectors, and the flow cell.
- Lower buffer molarity and organic modifiers percentage if applicable
3) peak tailing:
Peak tailing, where the analyte peak shows an extended tail, is a major issue that compromises quantification and the visual interpretation of chromatograms.
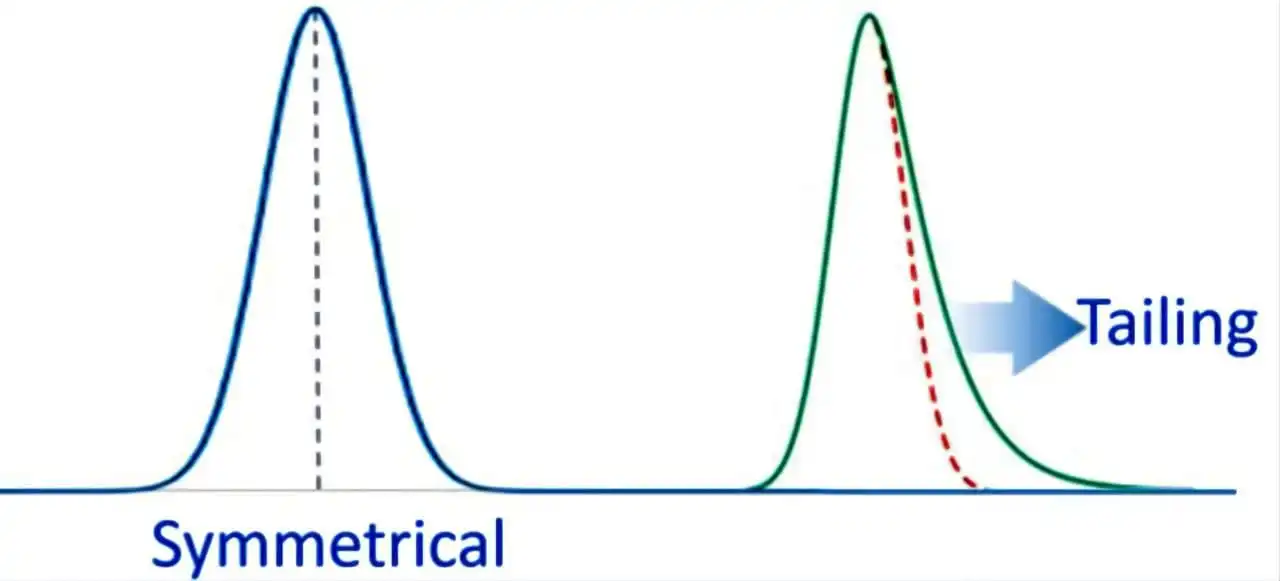
Causes:
- Stationary Phase Interaction: the main cause of peak tailing is its interaction with the stationary phase in more than one way: the usual nonspecific hydrophobic interaction as well as the secondary interaction with the residual polar ionised silanol group residing on the silica support surface (this is usually common with basic compounds having amines or other basic functional groups at ph >3 where the basic functional groups are ionised and silanol groups are not fully protonated). Also, if the stationary phase is not suitable for the analyte (e.g., strong ionic interactions, peak is too retained), it may cause retention and tailing. This is often seen in reversed-phase chromatography when the analyte interacts strongly with the stationary phase
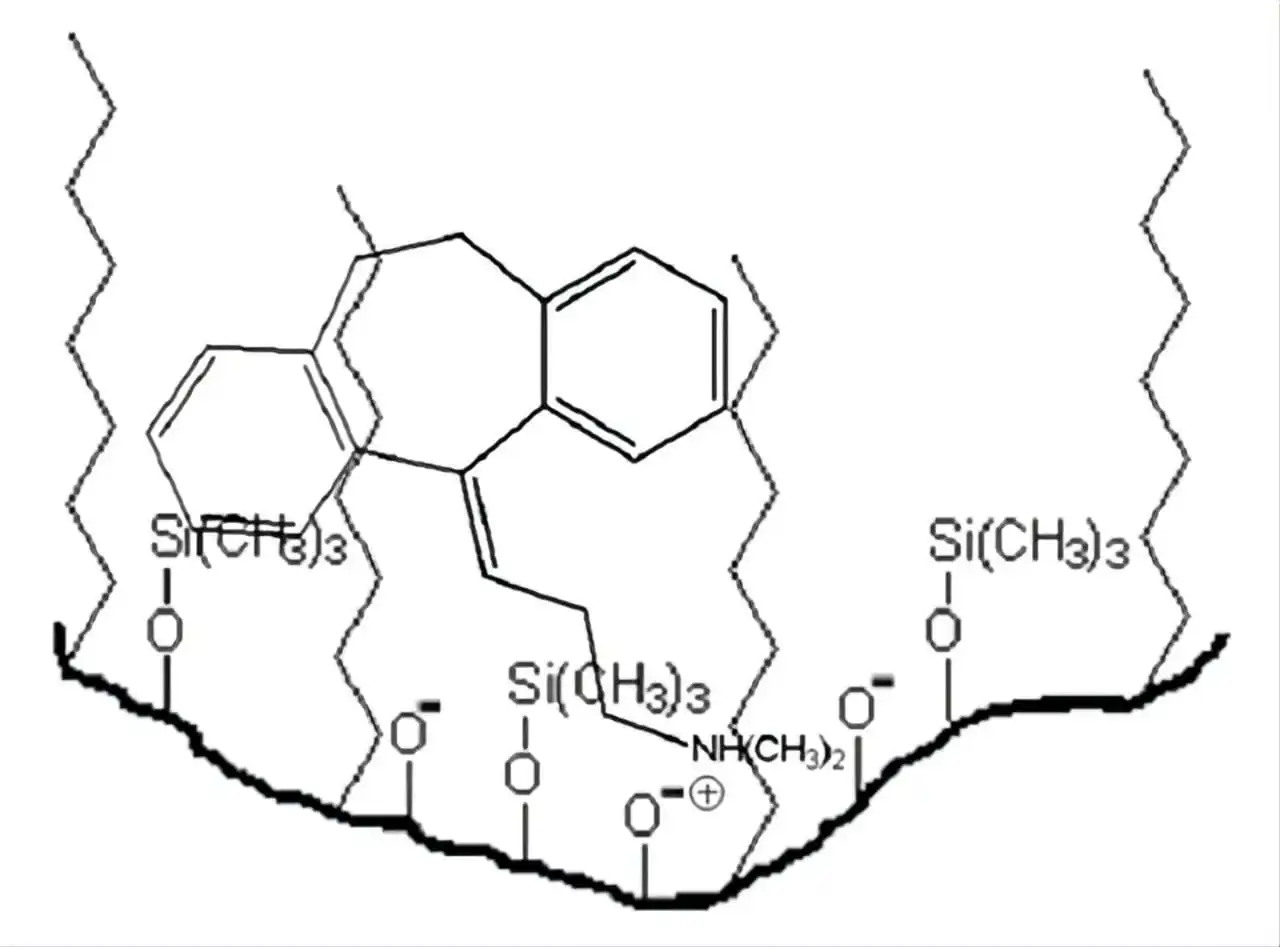
- Column Overloading: High sample concentration can result in peak tailing, particularly for substances with strong adsorption to the stationary phase.
- Column Contamination: If the column packing material is contaminated by residual sample components or solvents, this can result in asymmetrical peaks.
- Inadequate Mobile Phase: The pH or organic modifier concentration in the mobile phase may not be optimized, leading to incomplete elution or long retention times.
Solutions:
- Switch Stationary Phases: If the column’s stationary phase is unsuitable for the analyte, consider switching to a more appropriate phase (end-capped highly deactivated columns where silanol groups are masked) or using a different column length or diameter. Also consider chemical reagents in the mobile phase used to end-cap columns and deactivate the silanol group like triethylamine, trimethylchlorosilane (TMCS) and hexamethyldisilazane (HMDS).
- Work at high pH if possible, when analyzing basic compounds
- If column allows it (zorbax or hypersil columns), work at pH<3 to ensure full protonation of silanol groups
- Optimize Injection Volume: Reduce the sample concentration to prevent overloading the column.
- Use Gradient Elution: If a single solvent or mobile phase composition causes peak tailing, switching to gradient elution may help improve peak symmetry by modifying the mobile phase composition during the run.
- Increase Flow Rate: Increasing the flow rate slightly can reduce analyte interaction with the stationary phase, improving peak shape.
4) Peak Fronting:
Peak fronting occurs when an asymmetric peak is broader in the first half than the second half
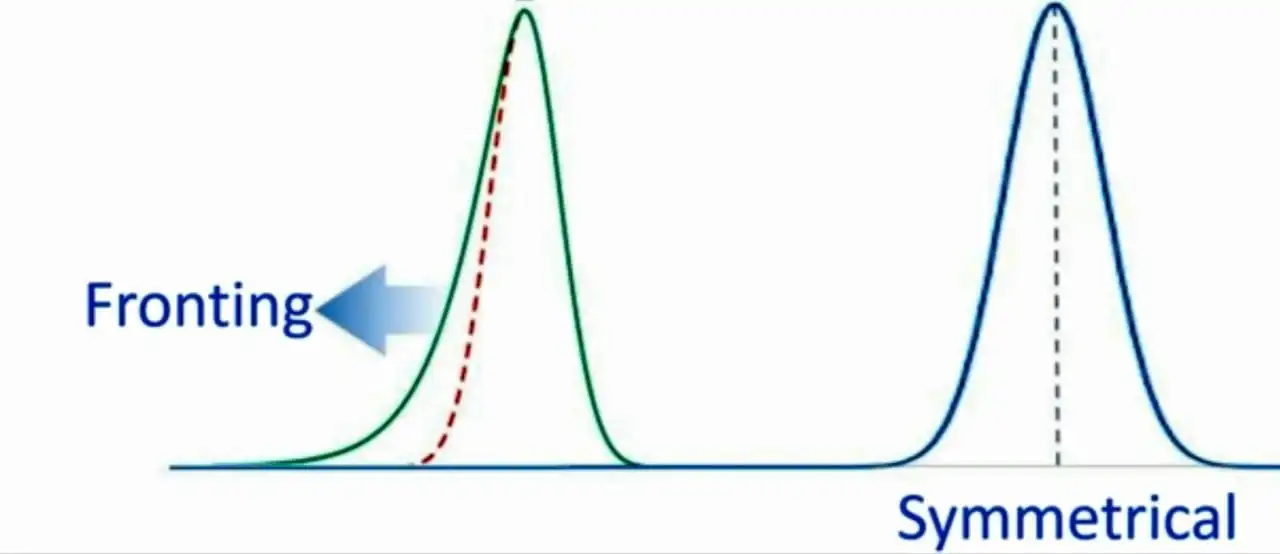
Causes:
- Sample overloading
- Solvation (solvent effect): occurs when the sample has higher affinity to the solvent than the mobile phase causing it to favour the solvent and stationary phase rather than being eluted with the mobile phase causing fronting of the peak
Solutions:
- Reduce injection volume
- Ensure the sample solubility in the mobile phase
5) Low Sensitivity or Weak Signal:
Low sensitivity is a common issue when analyzing trace analytes, leading to low peak heights or the inability to detect the analyte at the desired concentration.
Causes:
- Improper Detector Settings: The detector may be set to an incorrect wavelength (inappropriate wavelength) , gain, or integration settings, leading to reduced sensitivity.
- Column Degradation (aging and contamination): Over time, columns lose their ability to retain analytes, particularly in cases of irreversible adsorption or physical damage.
- Flow Rate Issues: Inconsistent flow rates due to pump malfunctions or leaks can reduce the signal strength.
- Leaks in the system: Leaks can cause a reduction in flow rate and detector response
Solutions:
- Optimize detector settings: Choose the correct wavelength based on the analyte’s absorbance characteristics and optimize gain settings to maximize sensitivity.
- Check for leaks: Inspect the entire system for leaks, especially in the pump, injector, and detector seals.
- Column regeneration or replacement: Regenerate the column by flushing it with solvents or replace it if needed.
- Check Flow Rates: Ensure the pump is delivering a consistent flow rate and that all system components are functioning properly.
6) Pressure Fluctuations:
- Fluctuating or high backpressure is one of the most common issues in HPLC. High-pressure fluctuations can affect flow stability, column performance, and even cause pump failure. These fluctuations may manifest as irregular retention times, erratic peaks, and difficulties with reproducibility.
Causes:
- Clogged Filters: The presence of particulate matter or precipitates in the mobile phase can block filters and frits, increasing resistance and causing pressure instability.
- Column Blockage: Columns may become blocked with sample residues, salts, or other contaminants, which increase pressure and decrease flow rate.
- Leaks: Leaks in the pump, injector, or detector flow paths can reduce system pressure, leading to unstable flow and inconsistent results.
- Poor Solvent Filtration: If the mobile phase is not filtered before use, particulates can accumulate in the system, contributing to pressure variations.
Solutions:
- Regularly Replace and Clean Filters: Ensure that inline filters, frits, and the pump head are regularly cleaned or replaced.
- Check for System Leaks: Inspect every connection, including the pump, injector, and detector, for possible leaks that may reduce system pressure.
- Mobile Phase Filtration: Filter all solvents through a 0.45 µm or 0.22 µm filter before use to prevent particulate buildup in the system.
- Column Backflushing: Use a backflush technique or clean the column periodically with appropriate solvents to remove any accumulated debris or contaminants.
7) Reproducibility Issues:
A lack of reproducibility can result in inconsistent results between runs or between different operators, which compromises the accuracy and reliability of the data.
Causes:
- Inconsistent mobile phase preparation: Variations in the mobile phase preparation, such as differences in solvent purity or ionic strength, can affect reproducibility.
- Temperature fluctuations: HPLC systems sensitive to temperature may experience variability in retention time and peak shape due to environmental changes.
- Operator error: Variations in injection volume or improper method execution can lead to inconsistent results.
Solutions:
- Standardize mobile phase preparation: Use high-quality solvents and maintain consistent mixing procedures for reproducibility
- Control environmental factors: Ensure that the system operates at a constant temperature and that the room conditions remain stable.
- Proper training: Regularly train operators to follow standard operating procedures (SOPs) to minimize human error.
conclusion
Troubleshooting in HPLC requires a deep understanding of the system components, method development principles, and potential issues that can arise during analysis. Addressing issues such as resolution, baseline noise, peak tailing, and sensitivity can optimize HPLC performance and ensure accurate, reproducible results. Regular maintenance, system checks, and method optimization are key to resolving common problems and maintaining system longevity.
References
- Snyder, L. R., & Kirkland, J. J. (2000). Practical HPLC Method Development (2nd ed.). Wiley.
- Matysik, F. M., & Skoog, D. A. (2010). Principles of Instrumental Analysis (6th ed.). Cengage Learning.
- Cech, J., & Lenz, E. (2009). Chromatographic Methods in Clinical Chemistry and Toxicology. Elsevier.
- Van Deemter, J. J., et al. (2009). HPLC and UHPLC for Practitioners. Springer.
- Guiochon, G., et al. (2006). Fundamentals of Preparative and Nonlinear Chromatography (2nd ed.). Academic Press.
- Rønsted, K. et al. (2011). Handbook of HPLC. Elsevier.
- https://www.elementlabsolutions.com/uk/chromatography-blog/post/peak-tailing-in-hplc#:~:text=The%20primary%20cause%20of%20peak,interactions%20with%20the%20stationary%20phase.
- https://www.acdlabs.com/blog/an-introduction-to-peak-tailing-fronting-and-splitting-in-chromatography/#:~:text=Several%20factors%20may%20cause%20peak,volume%20or%20the%20solute’s%20concentration.


