Abstract
High-performance liquid chromatography (HPLC) is an essential analytical tool in assessing drug products. HPLC methods should be able to separate, detect, and quantify the various drugs and drug-related degradants that can form during storage or manufacturing. The development of a valid analytical method is crucial during the process of drug discovery and development. Since high-performance/pressure liquid chromatography (HPLC) is the most commonly used technique for drug analysis, it will be our focus in this article. The purpose of this article is to dive into the details of the analytical method development for assay determination of drug substances; the choice of drug concentration, solvent, buffer, mobile phase, stationary phase, injection volume, flow rate, temperature, and wavelength.
Introduction
HPLC is a process of separating components in a liquid sample mixture, which is injected into a stream of solvent (mobile phase) flowing through a column packed with a separation medium (stationary phase). Sample components separate from one another by a process of differential migration as they flow through the column. The resolution is important and is dependent upon the extent of interaction between the sample mixture components and the stationary phase. The interaction of the sample mixture with mobile and stationary phases can be manipulated through different choices of both mobile and stationary phases.
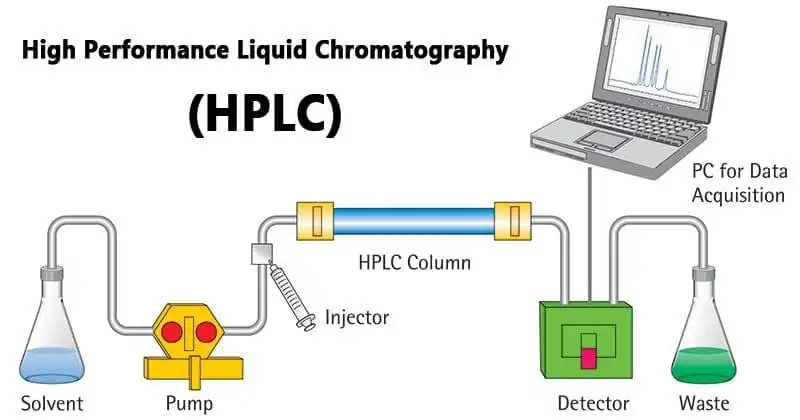
Figure 1: HPLC flow diagram
Analytical method development.
Steps involved in analytical method development are:
- Understanding the physico-chemical properties of the drug molecule.
- Selection of chromatographic conditions.
- Development of the approach to analysis.
- Sample preparation.
- Method optimization.
- Method validation.
1. Understanding of the physico-chemical properties of drug molecules.
For analytical method development, we need to study the drug properties like structure, solubility, polarity, pKa, pH, stability, and sensitivity to light and heat and most importantly its toxicity to ensure proper handling. Polarity is a physical property of a compound, it helps an analyst to decide the solvent and composition of the mobile phase. In a nonpolar covalent bond the electrons are shared equally between two atoms. A polar covalent bond is one which one atom has a greater attraction for the electrons than the other atom. The solubility of molecules can be explained based on the polarity of molecules. Polar, e.g. water, and non polar, e.g. benzene, solvents do not mix. In general, like dissolves like i.e., materials with similar polarity are soluble in each other.
2. Selection of chromatographic conditions.
Since most drugs are weak acids or weak bases, we usually use reversed-phase chromatography as the stationary phase is non-polar to be able to interact with the analyte of interest, and the mobile phase is polar, causing polar peaks to elute earlier than relatively non-polar peaks.
2.1.How to select a column?
The core of an HPLC system is the column. Changing a column will have the greatest effect on the resolution of analytes during method development. Generally, modern reverse-phase HPLC columns are made by packing the column housing with spherical silica gel beads, which are coated with the hydrophobic stationary phase. The stationary phase is introduced to the matrix by reacting a chlorosilane with the hydroxyl groups present on the silica gel surface. In general, the nature of the stationary phase has the greatest effect on capacity factor, selectivity, efficiency, and elution. Selecting the appropriate column for an application depends on:
- Stationary phase chemistry.
- Retention capacity.
- Particle size.
- Column measurements
There are various types of matrices, including:
Silica matrices are robust, easily derivatized, manufactured to a consistent sphere size, and do not tend to compress under pressure. Silica is chemically stable to most organic solvents and to low pH systems. One shortcoming of a silica solid support is that it will dissolve above pH 7. Therefore, to overcome this issue silica silica-supported columns have been produced for use in case of high pH values. The nature, shape, and particle size of the silica support affect separation. Smaller particles result in a greater number of theoretical plates, or increased separation efficiency. However, the use of smaller particles also results in increased backpressure during chromatography, and the column more easily becomes plugged.
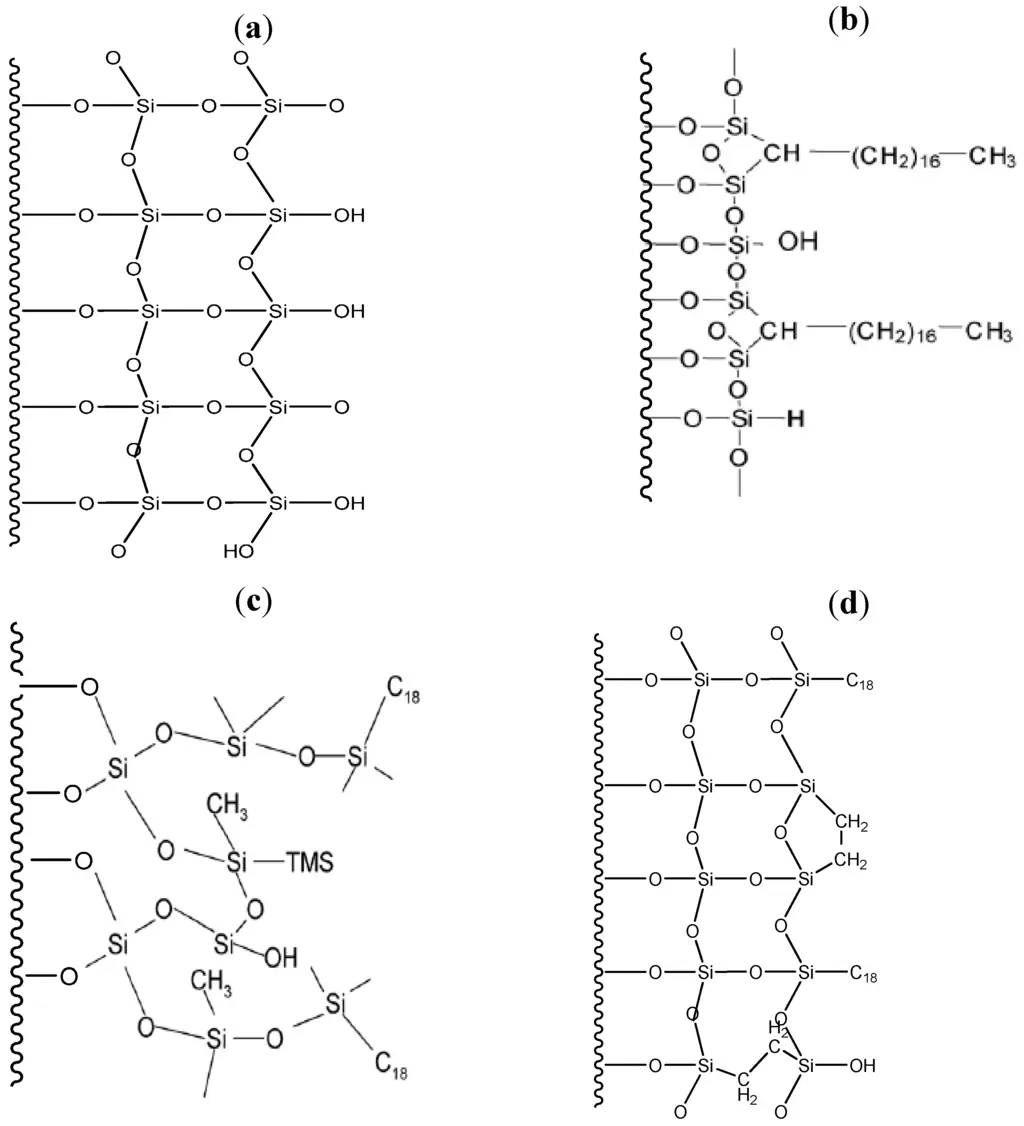
figure 2: Representative surface chemical structure of (a) bare silica (b) cogent bidentate C18 (c) XTerra MS C18; Trimethylsilane (TMS) endcapping shown (d) XBridge Prep C18
The major types of silica are:
- Alkyl bonded phases such as C8 and C18 (their major difference between C18 and C8 is that the hydrophobic molecules may be retained more on the C18 column than the C8).
- Amino.
- polar embedded (They are columns designed to have the same long chain of silica (C18) in addition to polar groups attached to the silica. Used with polar molecules)
- Pentafluorophenyl (PFP) (Meant for less hydrophobic molecules or polar compounds)
- Cyano.
- Non-bonded pure silica.
Octyl (C8) columns have wide applicability. This phase is less retentive than the C18 phases, but is still quite useful for pharmaceuticals,example: (Zorbax SB C8, Luna C8, and YMC Pack MOS).
Octadecyl (C18, ODS) columns are the most widely used and tend to be the most retentive for non polar analytes, examples include Zorbax SB C18, YMC Pack ODS and Luna C18. Xterra RP C18 and Zorbax Extend C18 columns have been formulated to tolerate high pH systems (pH >7, normally up to pH 11).
Varying the pH can affect selectivity and resolution of polar analytes, especially for ionizable compounds. Phenyl (Ph) columns offer unique selectivity from the alkyl phases and are generally less retentive than C8 or C18 phases. Phenyl columns are commonly used to resolve aromatic compounds. Examples include Zorbax SB Phenyl, YMC Pack Phenyl and Luna Phenyl Hexyl. Nitrile (CN or cyano) columns are polar and can be used for both reverse and normal phase applications. This phase is often used to increase the retention of polar analytes. Examples include Zorbax SB CN, Luna CN, and YMC Pack CN. The type of column chosen for a particular separation depends on the compound and the aim of analysis
Also, Column length greatly affects separation (As column length increases, peaks are better resoluted with higher plates) as well as particle size which generally affects selectivity of column; smaller particle size results in higher selectivity
2.2. Optimization of Mobile phase:
2.2.1 Buffer selection:
Most drugs in solutions are either in an ionised or non ionised form, unfortunately this may lead to peak splitting or forking, that’s why it’s crucial to have a salt solution (Buffer) to fix the pH away from drug’s pka to ensure that the drug is either completely ionised or non ionised . According to Henderson Hasselbach equation to ensure that most of the drug is in the ionised or non ionised form we must choose a pH that is far away from the pka of the drug by at least 2
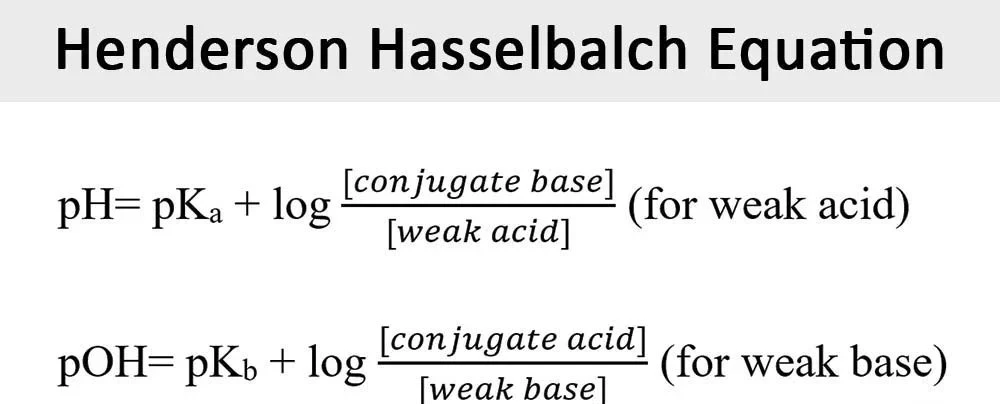
figure 3: henderson Hasselbalch Equation
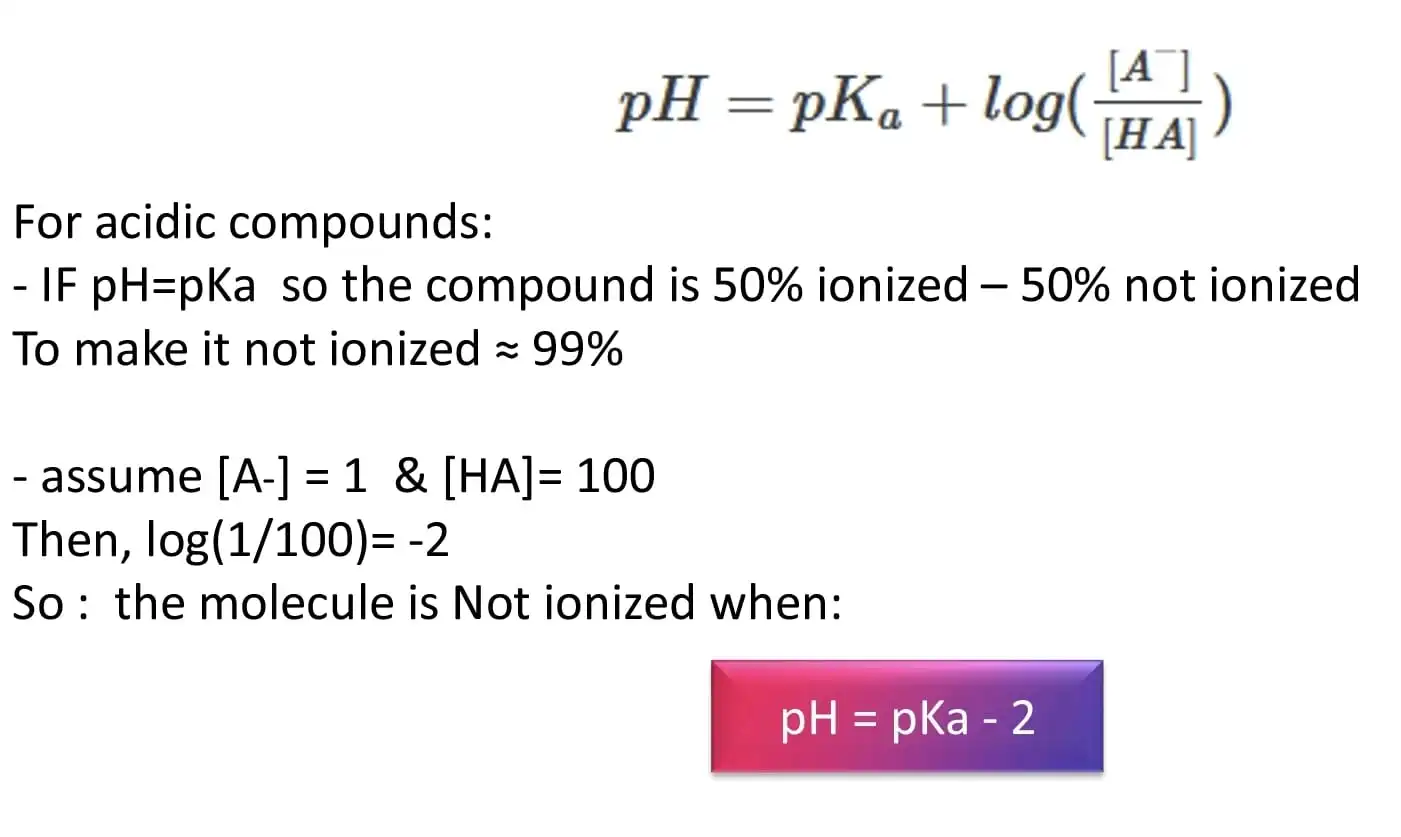
figure 4: pH selection
The closer the buffer pH is to the pKa of the salt used, the highest the buffering Capacity (The buffer can cover the pH change around its pka value). Salt selection is based on the desired pH range to be covered we choose the salt having a pka near the desired pH
If the peak keeps shifting from one injection to another, consider increasing buffer capacity by increasing the molarity of the salt used.
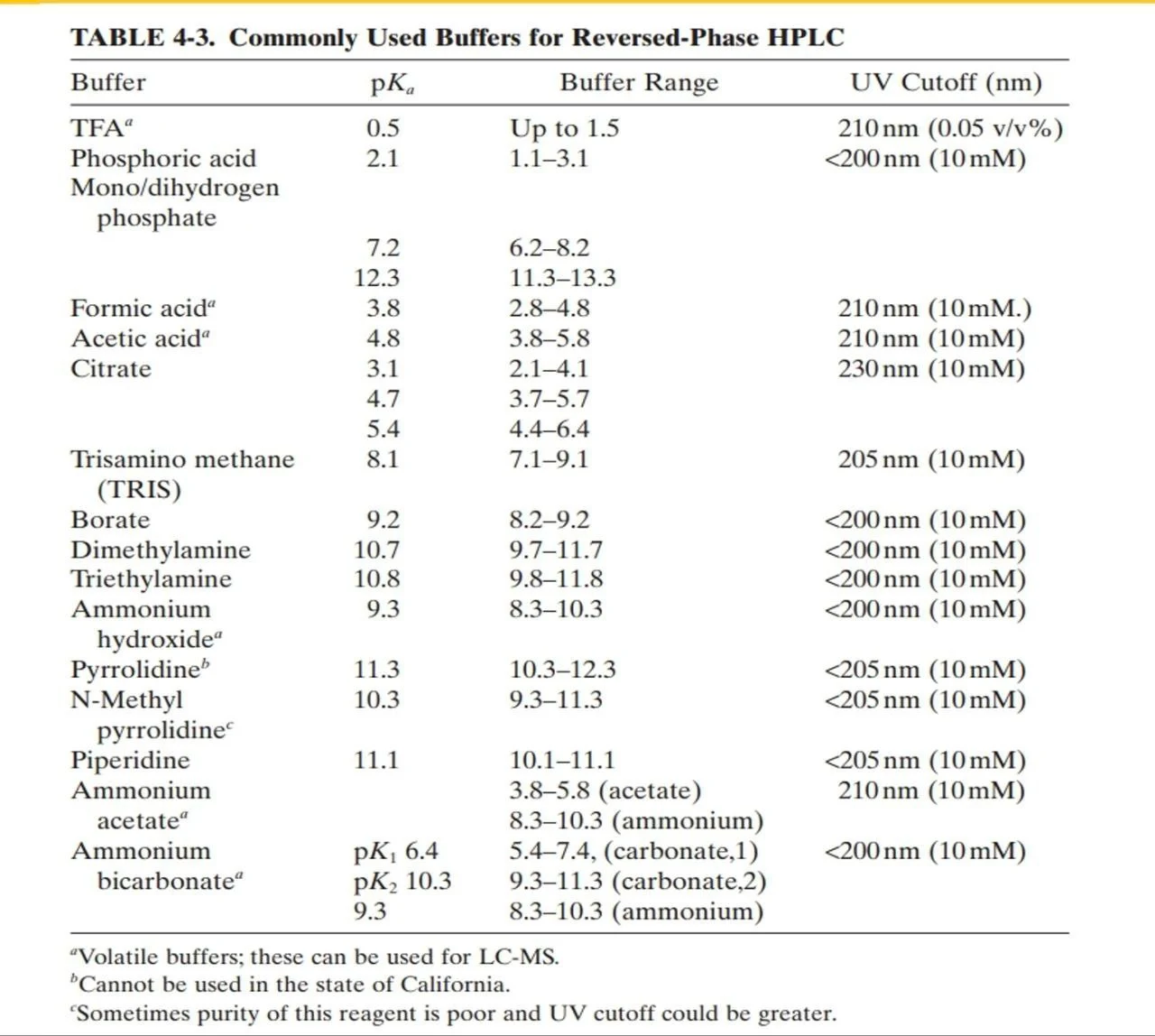
figure 5: HPLC buffers, pKa values and useful pH ranges.
Ion pairing reagent
If the molecule is not retained on the column, then we might need an ion pairing-reagents: These reagent molecules carry charges opposite to that of the analyte ions with which they are able to form electrostatic bonds. The pairs formed between the analytes and reagent ions behave like neutral, hydrophobic moieties that can be separated on C18 or C8 columns. These reagents include (alkylsulfonates like pentane, hexane,heptane, octane sulfonic acid and tetraalkylammonium salts for negatively charged molecules).
Ion pair chromatography is used for the separation of polar organic acids, bases and zwitterions as well as inorganic ions. These reagent molecules carry charges opposite to that of the analyte ions with which they are able to form
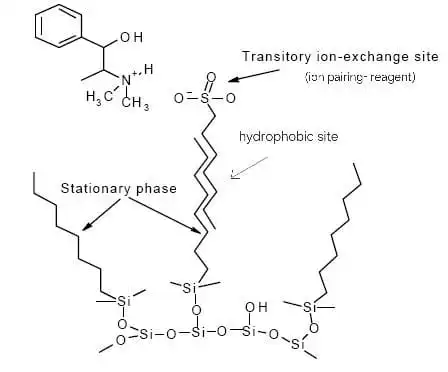
figure 6: ion-pairing reagent
2.2.2. Mobile phase ratio (Buffer to Organic):
The mobile phase impacts resolution, selectivity, and efficiency. Organic modifiers are responsible for eluting the hydrophobic compounds, which would be more retained on the column, so less polar solvents should be used. The most common solvents used: acetonitrile, methanol, THF, isopropanol, and hexane. The most commonly used organic modifiers are methanol and acetonitrile; acetonitrile has a higher elution power than methanol
A “Glass cylinder test” should be conducted to determine if the buffer will precipitate in the column/system when it is exposed to the highest organic concentration in the gradient.
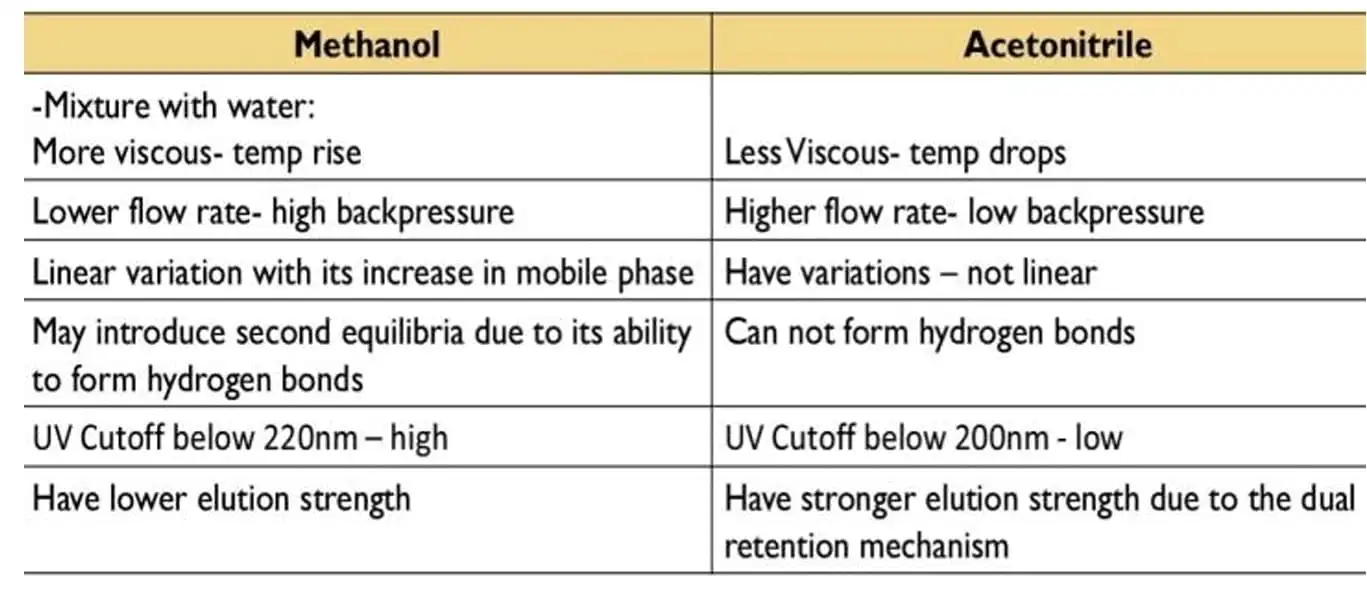
figure 7: Methanol vs. Acetonitrile
The elution could be:
Isocratic Elution (Same buffer to organic ratio throughout the run)
This examination makes isocratic separations more predictable, although the separation power (number of compounds which could be resolved) is not very high. The peak capacity is low, and the longer the component is retained on the column, the wider is the resultant peak.
Gradient elution (Buffer to organic ratio is changing throughout the run)
Gradient separation significantly increases the separation power of a system mainly because of the dramatic increase of the apparent efficiency (decrease of the peak width). The condition where the tail of a chromatographic zone is always under the influence of a stronger eluent composition leads to a decrease in the peak width. Changing Gradient: Gradient elution is employed for complex multicomponent samples or closely related molecules since it may not be possible all components eluted using a single solvent strength under isocratic conditions. When a gradient method is used, the column must allowed to equilibrate at the starting mobile-phase conditions prior to the next sample injection and the start of the next gradient run.
| HIGH VS LOW ORGANIC | High organic percentage | Low organic percentage |
| Advantages | -Faster elution. -It enhances peak shape. -It increases theoretical plates. | -The peak not retained enough, so it may cause closely related peaks to overlap |
| Disadvantages | -The peak is not retained enough, so it may cause closely related peaks to overlap | -Slower elution. -Results in broad peaks. -It decreases theoretical plates |
2.3.Temperature selection:
Column temperature control is important for long-term method reproducibility temperature affects selectivity. Target temperature in the range of 30 40 °C is normally sufficient for good reproducibility. Higher temperatures enhance peak shape and decrease
backpressure over the column, also decrease the viscosity of the mobile phase and allow for high flow rate, but take into consideration the maximum temperature for each column to avoid ruining the silica
2.4. Flow rate selection:
Higher flow rates may increase the analysis speed and enhance the peak shape, but they may affect the column if backpressure isn’t monitored
2.5. Injection volume selection:
Higher injection volumes allow detection and quantitation of small quantities of samples, but result in noisier baselines, which may affect the integration and detection of the peaks, especially if they have small UV absorbance. Also, when a very large volume of sample is injected into the HPLC column, the peaks begin to front more (peak symmetry factor < 1) due to sample overloading, and the retention time may decrease, resulting in a decline in column efficiency and separation resolution.
2.6. Selection of detector and wavelength:
UV-Visible detector:
UV-Visible detector: is flexible, allowing a double wavelength absorbance detector for HPLC. Used with materials that have absorbance in the UV region. The more π electrons there are, the more absorbance the molecule is going to have in the UV region. (Each molecule has a maximum wavelength of absorbance.) For a less noisy baseline, it’s better to choose a wavelength above the cut-off point of the mobile phase and
diluent.
If the peak keeps shifting from one injection to the other, then detection must be at the isosbestic point: an isosbestic point is a specific wavelength, wavenumber, or frequency at which the total absorbance of a sample does not change during a chemical reaction or a physical change of the sample.
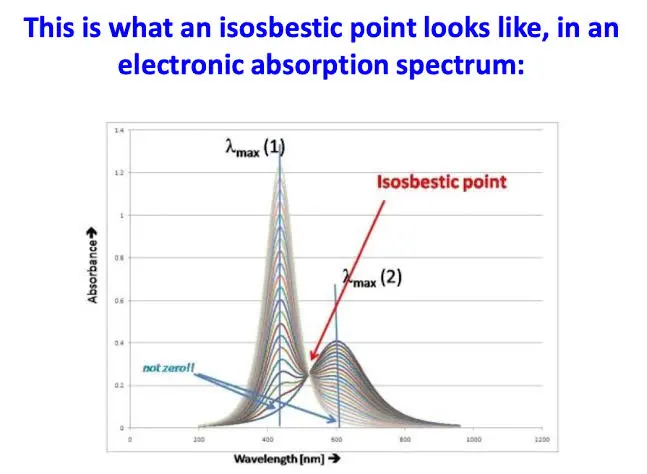
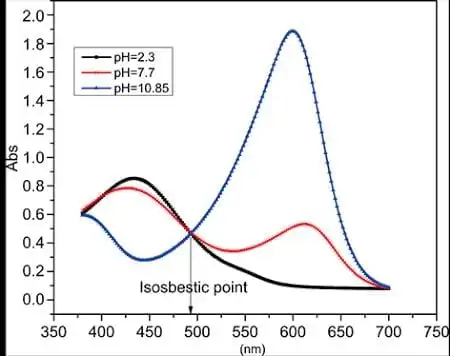
figure 8: isosbestic point
Refractive Index (RI):
Detector offers high sensitivity, strength, and reproducibility, which makes this detector the perfect solution for the investigation of parts with restricted or no UV absorption.(polymers)
Diode array detector:
Diode array detector used when detecting unknown compounds is a must or developing a related analysis method, due to its applicability to cover the whole spectrum of UV region, or checking the purity of a specific peak
Multi-Wavelength Fluorescence Detector:
Multi-Wavelength Fluorescence Detector. It operates based on the principle of measuring the fluorescence emitted by certain compounds when they are exposed to excitation light (Xenon lamp). Used when the target molecule has little or no UV absorbance
2.7.Diluent selection:
Ideally, the solvent would match the mobile phase to avoid any solvent effect ( disturbance of peak shape). If the analyte is more soluble in the diluent than the starting eluent composition, the compound will tend to reside in the “solvent plug” being injected onto the column, and a peak fronting or skewing may occur. The selection of diluents is based on the solubility of the analyte. The analyte must be soluble in the diluents and must not react with any of the diluent components. The diluent should match the starting eluent composition of the assay to ensure that no peak distortion will occur, especially for early eluting components.
3. Developing the approach for analysis:
While building up the analytical method on RPHPLC, the initial step that is pursued, the determination of different chromatographic parameters like choice of mobile phase, choice of column, choice of flow rate of mobile phase, and choice of pH of mobile phase. These parameters are chosen based on preliminaries and pursued by considering the system suitability parameters (according to the ICH guidelines)
Typical parameters of system appropriateness are:
- Capacity factor (K prime) >2
- The hypothetical plates ought to be more than 2000.
- The tailling factor ought to be under 2,
- The resolution between 2 peaks ought to be more than 2,
- % R.S.D. of the zone of analyte peak in standard chromatograms ought not be more than 2.0 %
4.Sample preparation:
Sample preparation is a basic advance of method development. For instance, the analyst must examine if centrifugation (determine the optimal rpm and time), shaking as well as filtration of the sample, is required to ensure maximum extraction of the analyte of interest by the method, particularly if there are insoluble segments in the sample. The goal is to show that the sample filtration does not influence the systematic outcome because of adsorption.
5.Method Optimization:
The experimental conditions should be optimized to get the desired separations and sensitivity after getting appropriate separations. Stability indicating assay experimental conditions will be achieved through planned/systematic examination parameters, including pH (if ionic), mobile phase components and ratio, gradient, flow rate, temperature, sample amounts, Injection volume, and diluent solvent type.
Conclusion
This review describes the general technique of HPLC method development. The general approach for the development of the method for the separation of pharmaceutical compounds was discussed. The knowledge of the pKa, pH and solubility of the primary compound is of utmost importance before the HPLC method development. Knowing pH can help discern the ionizable nature of the other impurities (i.e., synthetic byproducts, metabolites, degradation products, etc.) in the mixture. Selection of buffer and mobile phase composition (organic and pH) plays a dramatic role in the separation selectivity. Final optimization can be performed by changing the temperature, gradient slope, and flow rate as well as the type and concentration of mobile-phase modifiers.
References:
- Hemdan, Sokaina & Mansour, Asma & Ali, Fatma. (2019). Importance of isosbestic point in spectroscopy: review. 62. 1 21. 10.37376/1571 000 062 004.
- Azim, Md & Moloy, Mitra & Bhasin, Parminder. (2015). HPLC METHOD DEVELOPMENT AND VALIDATION: A REVIEW. International Research Journal of Pharmacy. 4. 39 46. 10.7897/2230-8407.04407.
- Bhardwaj, Dr. Santosh. (2015). A Review: HPLC Method Development and Validation.
- Srividya Kailasam, PhD. (2022).Ion Pair Chromatography How IPC Works, Strengths, Limitations and Applications, Technology networks
- bhaskar napte. (2023, October 20). Methanol vs. Acetonitrile: Which Solvent is Better for Chromatography? Pharmagrowthhub.
- Methanol vs. Acetonitrile: Which Solvent is Better for Chromatography?
- Sigma Aldrich. (2024). NMR Chemical Shifts of Impurities. Merck, 1(1).


