In our nature, No trait, No capability, Nothing ever… Fits all.
Everything evolves to meet specific needs, and when talking about HPLC detectors for chemical analysis, we should keep in mind the physicochemical characteristics of each sample. Each difference in a single property could cause a shift to another whole mechanism of detection, which would take us on a long explorative scientific journey through the different types of detectors and their different capabilities.
Whether it’s UV, fluorescence, refractive index, or mass spectrometry, each detector uniquely measures what matters most for the assigned task.
Types of Detectors
Let’s have an overview of the 2 main types of LC detectors.
| Bulk-property detectors | solute-property detectors |
| Functions mainly towards mobile phase properties, such as differential refractive index, density and Di-electric constant that is modulated by the presence of solutes | Functions mainly towards some properties of analytes, such as UV-vis absorbance, compound’s fluorescence, or diffusion current, that is not a property of the mobile phase |
The most widely used detectors for LC are based on the absorption of UV or visible radiation. Fluorescence, refractive-index, and electrochemical detectors are also widely used. Mass spectrometry (MS) detectors are now of significant use. Many absorption detectors are devices that utilize a double beam, where one beam goes through the sample cell and the second beam is a reference beam. Such LC/MS systems can greatly aid in identifying the analytes exiting from the HPLC column
UV-Visible Absorption Detectors
Principle: Beer-Lambert’s law
In UV-visible spectroscopy, the Beer-Lambert’s law forms a direct relation between the absorbance of a sample matrix and its corresponding concentration and the path length of the light beam. it tells that the quantity of light absorbed by a sample matrix is directly proportional to both the sample concentration and the distance the light travels through the matrix.
UV Absorption Detectors with Filters
The earliest absorption detectors were filter photometers with a mercury lamp as the source. Most commonly, the intense line at 254 nm was isolated by filters; with some instruments, lines at 250, 313, 334, and 365 nm could also be used by substitution of filters. This type of detector is restricted to solutes that absorb at one of these wavelengths. several organic functional groups and a number of inorganic species exhibit broad absorption bands that encompass one or more of these UV wavelengths.
Absorption Detectors with Scanning Capabilities
Most HPLC manufacturers offer detectors that consist of a scanning spectrophotometer with grating optics. Some detectors are limited to UV radiation; others utilize both visible and ultraviolet radiation. Several operational modes can be chosen. For example, the entire chromatogram can be obtained at a single wavelength; alternatively, when eluent peaks are sufficiently separated in time, different wavelengths can be chosen for each peak. Here again, computer control is often used to select the best wavelength for each eluent. Where entire spectra are desired for identification purposes, the flow of eluent can be stopped for a sufficient period to permit scanning the wavelength region of interest.
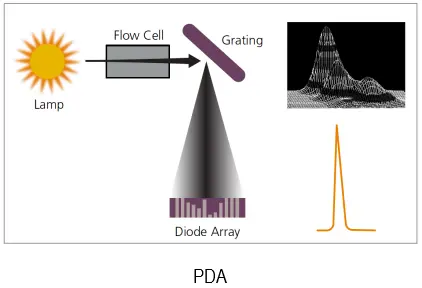
An illustrative Diagram of the scanning property of PDA
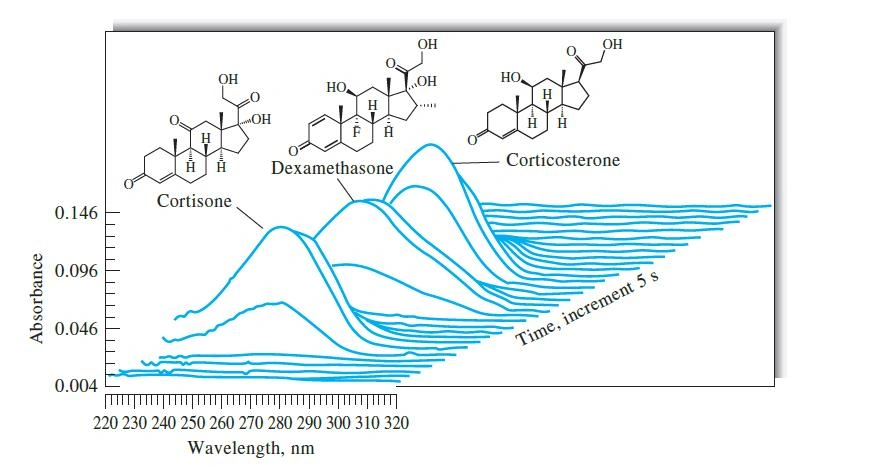
UV spectra for absorption of the eluent from 3 steroid mixtures taken at 5-second intervals. (Source: Agilent Technologies)
But what do you think the reason is?
Signal-to-noise is usually better at longer wavelengths due to a reduction in noise from the mobile phase and impurities
Have this example for illustration:
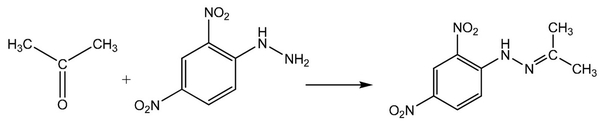
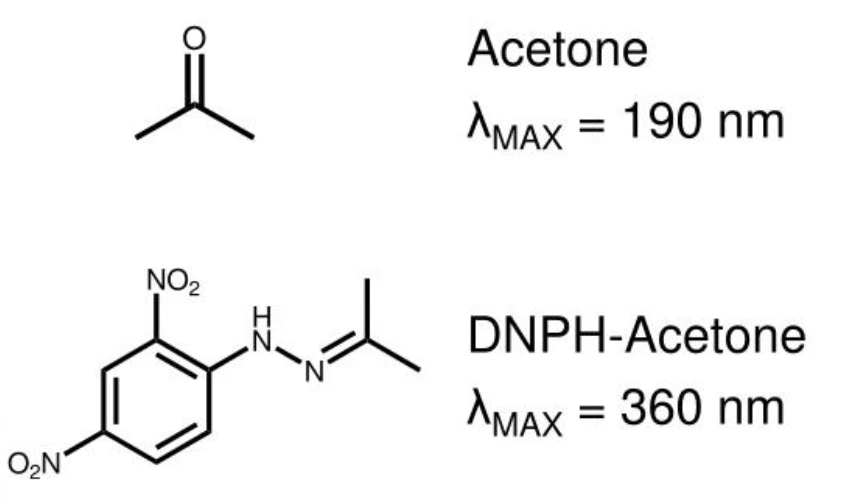
What do you think happened here?
Derivatization of Acetone into complex enabled its detection at a much higher wavelength, thus, clearer detection in presence of other impurities and excipients.
And as we mentioned, the absorbance of impurities and solvents, let’s have a look at the absorbance of commonly used organic solvents
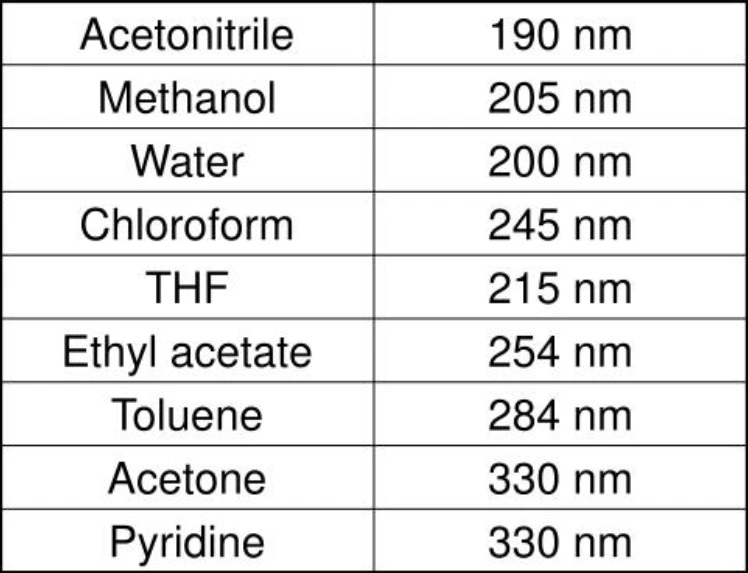
Your solvent of interest isn’t in the list? See this detailed list of UV-Absorbance of solvents
Now, let’s walk through another type of detector..
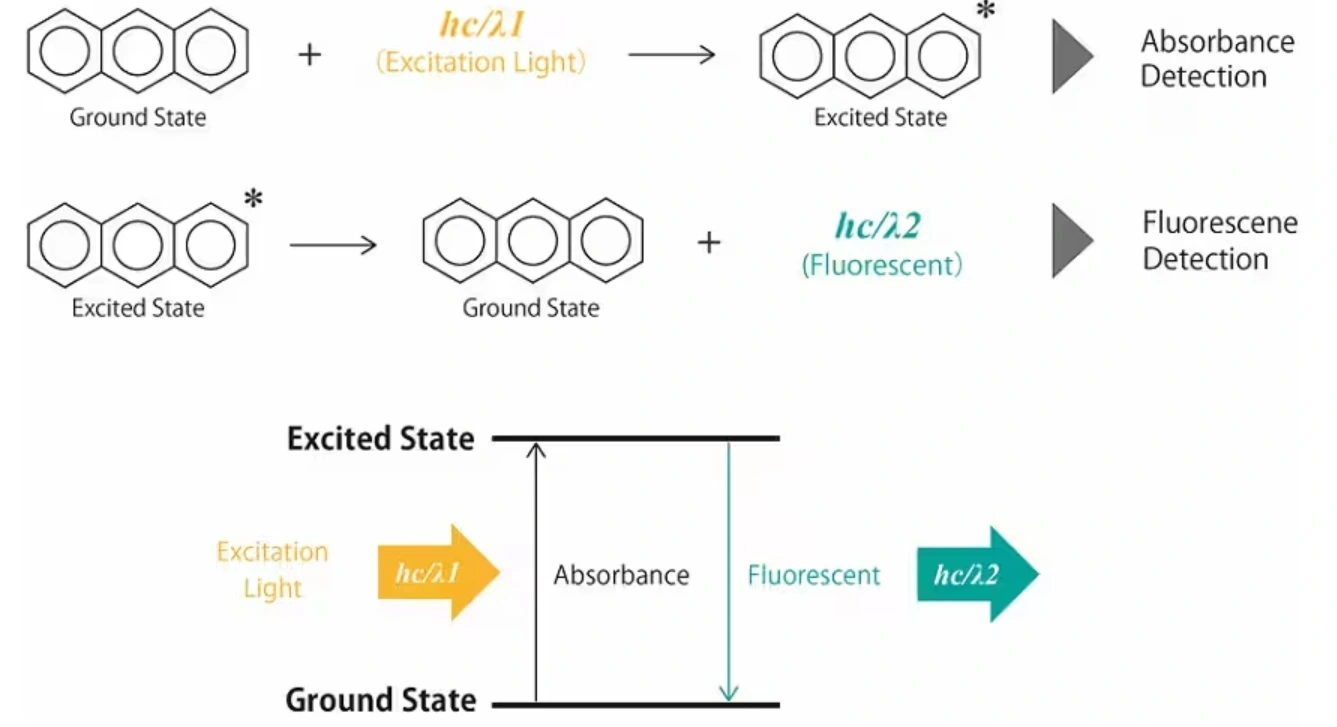
The next Detector is With greater sensitivity and selectivity over UV-Vis, but here is the game-changer… the analyte must fluoresce.
Fluorescence Detectors
How does it work??
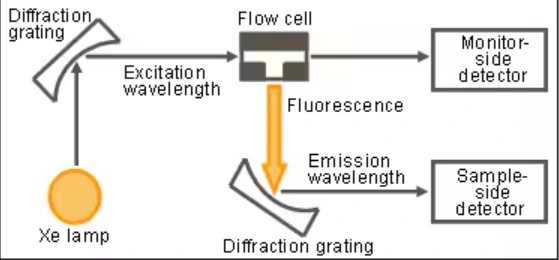
Fluorescence detector measures the intensity of this emitted fluorescence, providing information about the concentration of the fluorescent compound in the HPLC eluent. Two monochromators are used in FLD, the first selects the excitation wavelength and the other selects the emission wavelength
Limitations of Fluorescence Detector:
- Limited scope: Not all compounds are fluorescent, so derivatization may be needed.
- Can be sensitive to temperature: Some FLDs benefit from temperature control to maintain stability
Molecules with fluorescence properties are, in many cases, detected in such materials:
- Pharmaceuticals
- natural products
- clinical samples
- petroleum products.
Often, the number of fluorescing species can be enlarged by preliminary treatment of samples with reagents that form fluorescent derivatives. For example, dansylchloride (5-dimethylamino-naphthalene-1-sulphonyl chloride), which reacts with primary and secondary amines, amino acids, and phenols to give fluorescent compounds, has been widely used for the detection of amino acids.
To know more about the sample path inside the detector, you’d want to watch that informative video.
Refractive Index Detectors
Principle
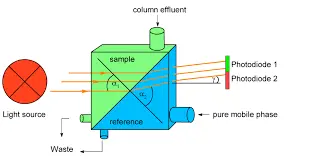
Refraction is when the light bends upon passing through medias with different refractive index, the difference in refractive index between the mobile phase and the sample allowing for the detection of analytes that may not absorb UV light.
The detector uses 2 beams of light: reference beam and sample beam, There is reference cell and a sample cell, you first purge the reference cell with mobile phase for a considerable amount of time till a stabilized readings are observed, then a valve is utilized to direct the sample out of the column to the sample cell, light will diffract differently through the two detector cells, The difference in refractive index between the two beams is converted into a signal that is displayed as a peak on the chromatogram.
Limitations:
- Sensitivity: They are less sensitive than other detectors like UV detectors and may require higher concentrations to be detected.
- Temperature Dependence: The refractive index is temperature-dependent, so temperature control is crucial for stable baselines.
- Gradient Elution: RI detectors are not typically suitable for gradient elution, as the refractive index of the mobile phase changes over time.
Applications of RI Detectors
- Polymer Analysis: RI detectors are widely used in polymer analysis, where they can detect polymers of various molecular weights and compositions.
- Sugar and Carbohydrate Analysis: They are effective for detecting sugars and carbohydrates, which often lack UV absorption.
- Other Applications: They are also used in the analysis of alcohols, lipids, and other compounds with weak UV activity.
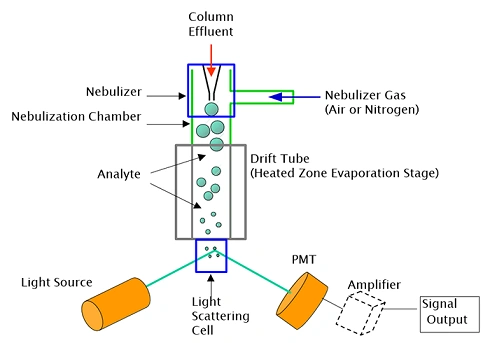
Evaporative Light Scattering detector
It is a type of detector used in High-Performance Liquid Chromatography (HPLC) and other chromatography techniques to detect non-volatile compounds in a volatile eluent, in other words, analytes with lower volatility than the mobile phase
Principle
ELSD detects compounds based on their ability to scatter light. It works by nebulizing the eluent, evaporating the mobile phase, and then shining a light source on the remaining analyte particles.
- Nebulization: The eluent is converted into a fine mist using a nebulizer and an inert carrier gas, like nitrogen.
- Evaporation: The mist is passed through a heated drift tube, where the mobile phase solvent evaporates, leaving behind the analyte particles.
- Light Scattering: The analyte particles after the evaporation process are exposed to a light source, and the scattered light is detected by a photodiode.
- Detection: The quantity of scattered light is in direct proportionality with the concentration of the sample.
Applications of ELSD
- Detect sugars
- advanced Fatty acids
- phospholipids
- vitamins
- amino acids
- triglycerides
- steroids
Mass Spectroscopic detector
Firstly, we have to get an ions source that generates ions at atmospheric pressure, Electrospray ionization (ESI) is a common ionization technique, then a mass analyzer to analyze these ions, finally a detector to detect them, as chromatographic peaks elute from the HPLC column and transfer to the ion source, two main processes occur. First, the source of ions releases charged molecules or ions, and second, the mobile phase is removed. Once the ions are produced, they are extracted from the ion source and headed to the mass analyzer. Ions in the sample are then filtered according to their mass to charge ratio by the quadrupole mass analyzer before detection.
Advantages:
- MS can distinguish between compounds with similar retention times based on their mass.
- Molecular weight information — Confirmation and identification of known and unknown compounds.
- Facilitated method development — Provides rapid identification of separated analytes without retention time verification.
Unlock the principles of precision, explore our full guide on HPLC Method Development, and take your analytical skills to the next level.
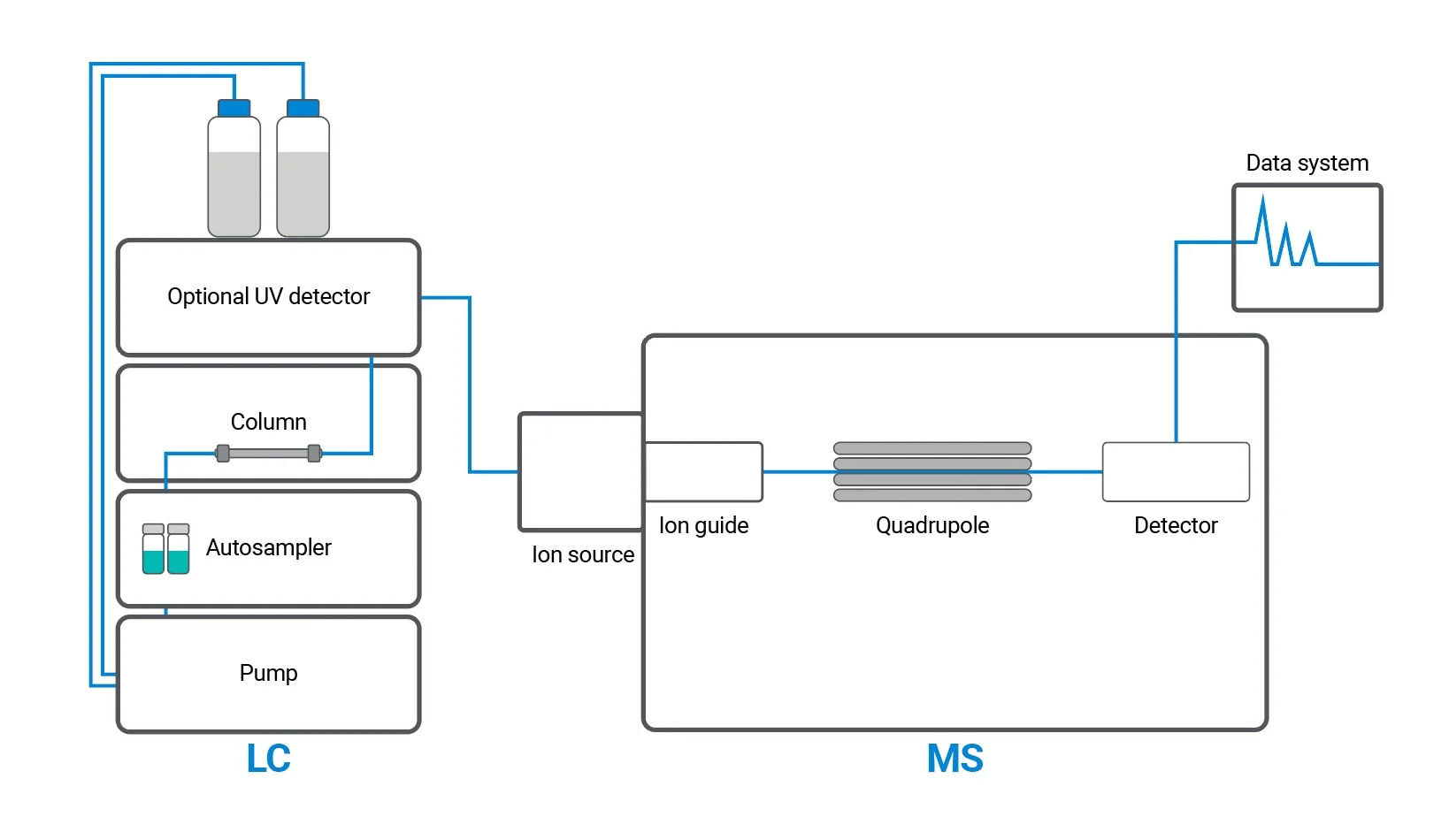
Discover how Liquid Chromatography–Mass Spectrometry (LC-MS) is unlocking the secrets of molecules. Read the full article now and dive into the science behind this powerful tool.
Electrical conductivity detector
Electrical Conductivity detector (CD) is widely used as a detector for ion-chromatography. It detects changes in electrical conductivity. Simply, Electrical conductivity is how much a substance can conduct electricity. When the sample gets out of the column and contains ion components, the ion concentration increases, making it easier for electric current to flow, i.e., thus, resulting in higher electrical conductivity.
The easiest form of ion chromatography is non-suppressed conductivity. In this method, the mobile phase has no UV absorbance, weak organic acid. Detection limits are high enough that the method is useful for the higher concentrations of anions in wastewater. The detector is positioned directly after the mobile phase elutes out of the column. The conductivity detector detects ions in the mobile phase and cations and anions in the sample. This technique enables you to perform ion chromatography using a normal HPLC.
After going into details about the types and principles of HPLC detectors, let’s answer and reflect on those questions:
- How to choose the right detector??
- How do we actually want the detector to perform??
- What do we need as strength points that are going to help the routine analysis??
The ideal desired properties of a detector are
- High sensitivity
- Analyte properties: the properties and nature of the analyte being detected, such as its UV-absorption, polarity, or fluorescence properties, can help decide which detector is most suitable.
- Uncomplicated and manageable operation and use.
- Observable linearity of response.
- The least Baseline noise.
- Stability and reproducibility.
- Dynamic Range (the range of concentration it can detect)
- Adaptability to different flow rates and pressures.
References
- https://www.chromatographyonline.com/view/mass-detection-chromatographers
- D. A. Skoog, F. J. Holler, and S. R. Crouch, Principles of Instrumental Analysis, 7th ed., Belmont, CA: Brooks/Cole
- D. A. Skoog, F. J. Holler, and S. R. Crouch, Fundamentals of analytical chemistry, 9th ed
- https://www.shodex.com/en/kouza_new/detector.html#anc-15-03


