High-Performance Liquid Chromatography or high-pressure liquid chromatography ( HPLC) is one of the most powerful and widely used analytical techniques in chemistry, biochemistry, and pharmaceutical sciences. It provides unparalleled resolution, speed, and sensitivity, making it a crucial tool in research and quality control; it ensures the purity of drugs and helps analyze complex biological samples. Whether in the pharmaceutical industry for drug testing, environmental science for pollutant detection, or food chemistry for contamination analysis, HPLC plays a pivotal role in separating and quantifying chemical substances.
What is HPLC?
HPLC is a chromatographic technique used to separate components in a mixture. It uses a liquid mobile phase to carry a sample (often in a liquid state) through a column packed with a solid stationary phase (Usually silica). It uses high pressure to push the mobile phase through the column. The sample components interact differently with the stationary phase, causing them to separate as they pass through the column. The separated components are then detected, analyzed, and quantified, often by ultraviolet (UV) absorption or other methods. (fig.1)
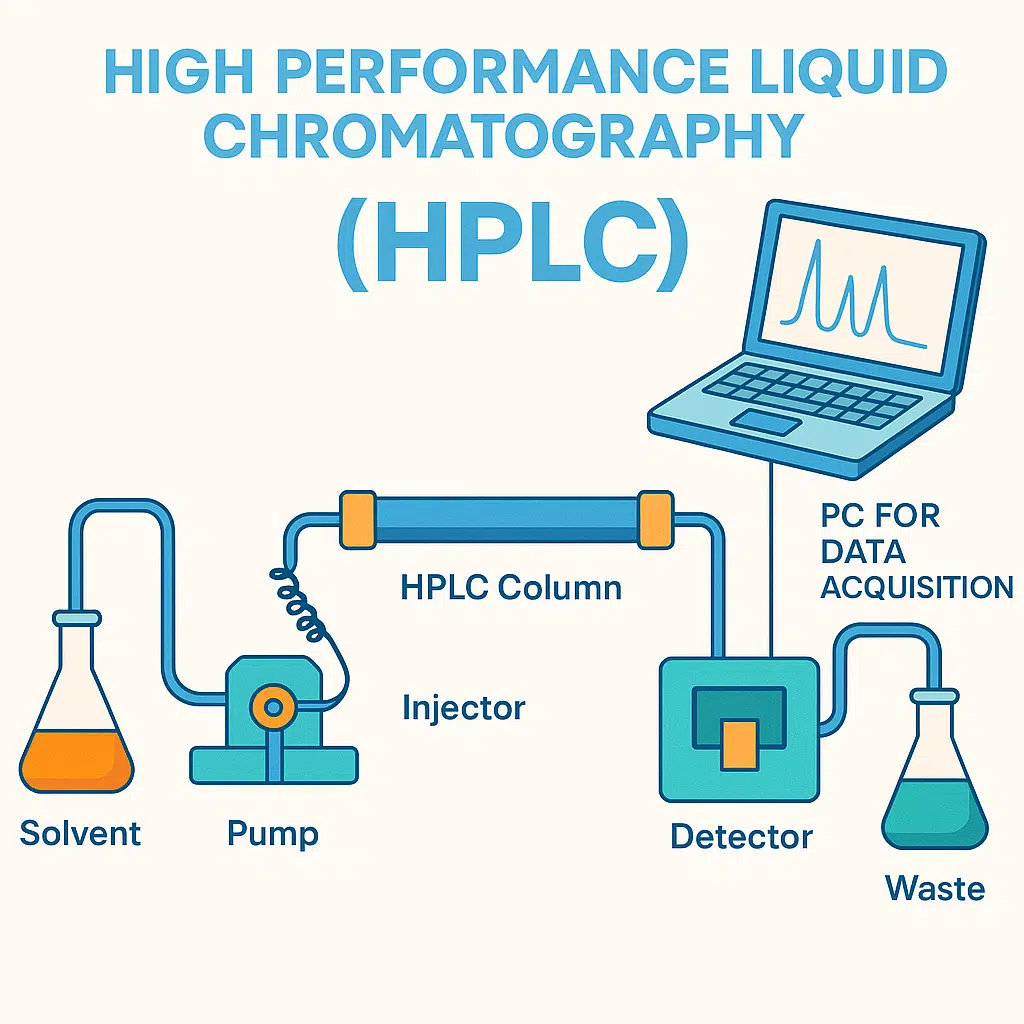
Figure 1: HPLC diagram
Basic Components of HPLC
Mobile Phase
- This is the solvent or mixture of solvents used to carry the sample through the column. The mobile phase can be aqueous, organic, or a mixture of buffer and organic modifier, depending on the sample’s chemical properties.
- Buffer: It’s a mixture of weak acid and its conjugate base or a weak base and its conjugate acid in a solution to resist any minor change in pH when a small amount of Acid or base is added.
➢Why control pH?
- The retention of ionisable compounds is sensitive to and dependent on the mobile phase pH, so it’s crucial to choose a pH where the compound is either completely ionised or completely non-ionised to avoid peak splitting, forking, or sometimes the appearance of 2 peaks for the same compound. To do so, the buffer pH must be at least 2 pH units above or below the compound’s pKa; acids are completely ionised at 2 pH units above its pKa and completely non-ionised at 2 pH units below its pKa, and vice versa for the base. The non-ionised form is less polar, thus more retained on reversed-phase columns; however, the ionised form is more polar (hydrophilic) and less retained on reversed-phase columns.
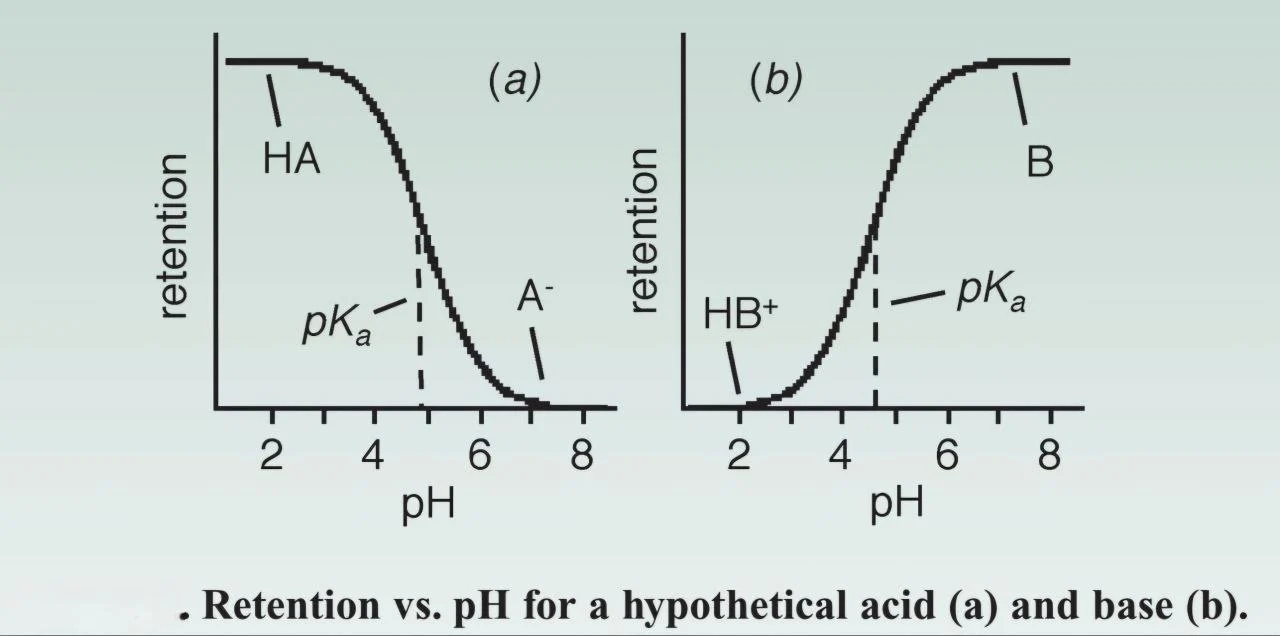
Figure 2: Retention VS pH for a hypothetical acid and base
- A buffer is at its highest capacity and most effective when used within +-1 pH unit of its pKa, but it still has adequate buffer capacity within +-2 pH units of its pKa (Figure 3).
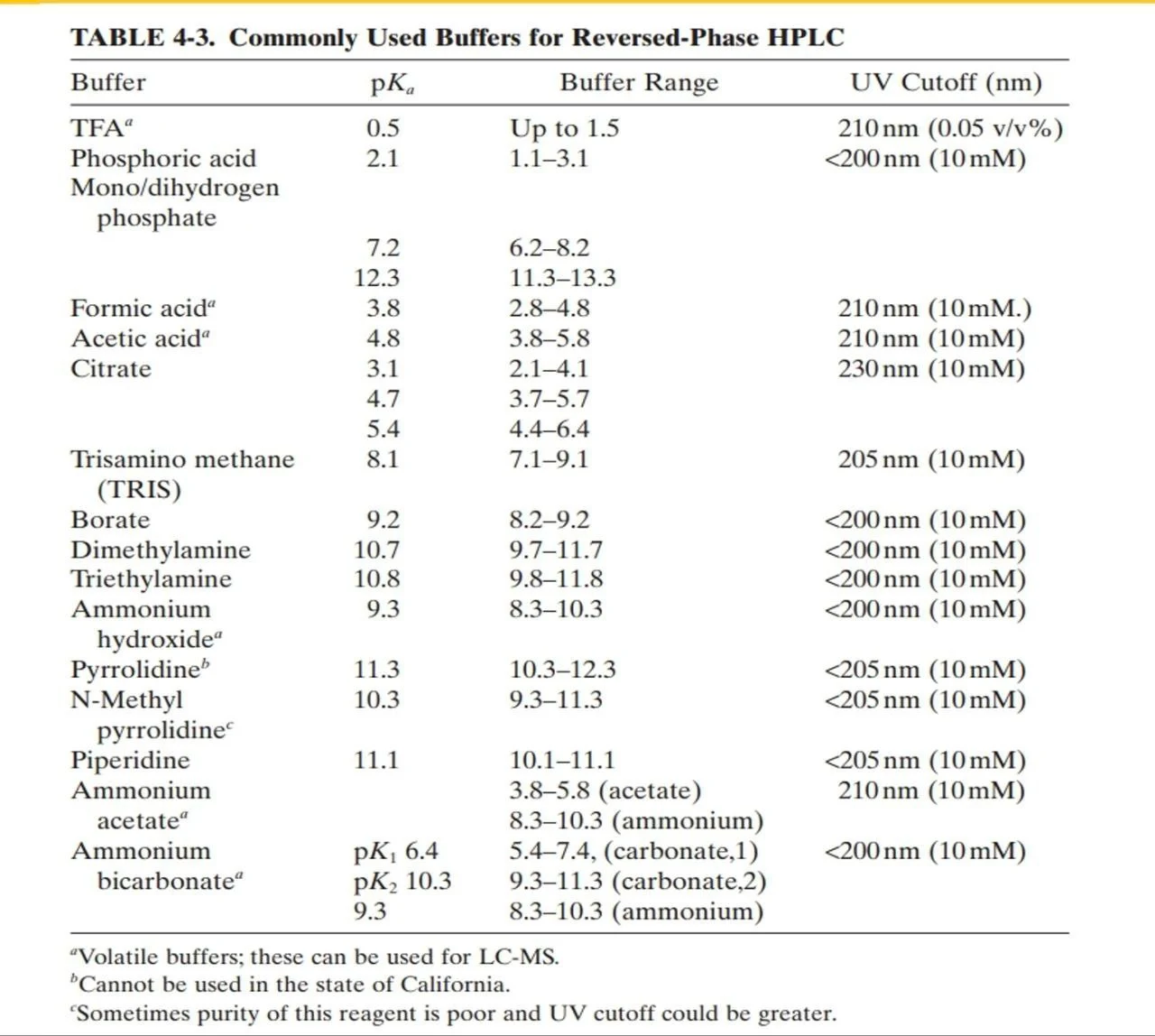
Figure 3: Buffers and their pKa and pH ranges
- Phosphate and acetate buffers and practically used more often practically as they have a UV cutoff <220, so they can be easily used at wavelengths below 220 without interference in peak purity.
Organic modifiers
- In reversed-phase systems, the aqueous mobile phase has weak elution power since most drugs are relatively non-polar, so organic modifiers are needed to give preference to the sample over the mobile phase
- Most commonly used organic modifiers are acetonitrile, methanol, and THF, with acetonitrile having the highest elution power (Figure 4)
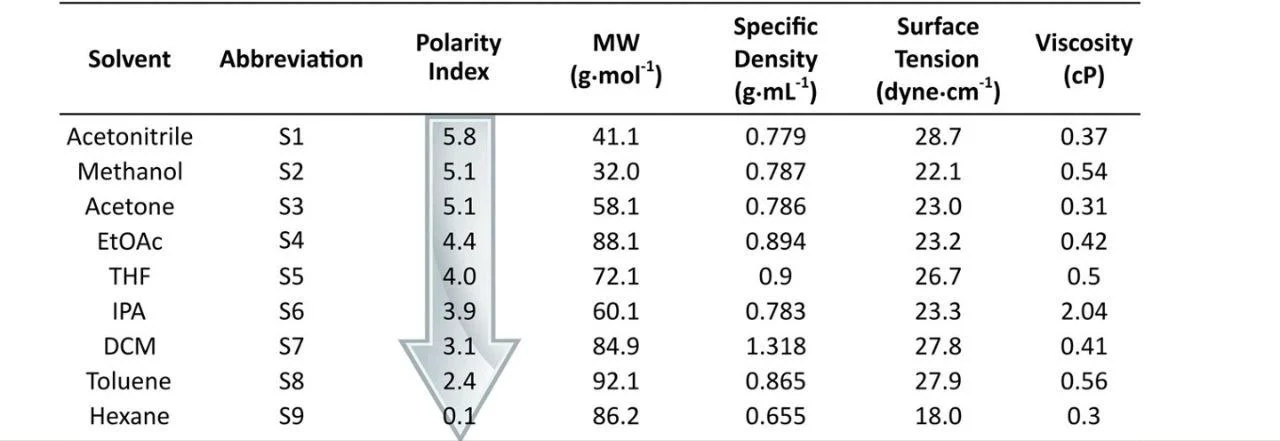
Figure 4: Organic modifiers and their elution power
Pump
- The pump delivers the mobile phase under high pressure through the system and is one of the most critical components. It ensures that the solvent is consistently delivered at a controlled flow rate. Common pumps include reciprocating pumps, which provide continuous flow. HPLC pumps are designed to work under high pressure, which allows the use of small particle size columns.
Column (stationary phase)
- The column is the core of the HPLC system. It is packed with a stationary phase, typically made of silica particles which are either unmodified or chemically bonded to hydrophobic or polar functional groups. The column is where the separation of the sample components occurs, It is usually made of stainless steel. Columns differ in length, internal diameter, pore size, particle size, carbon load, surface area, and chemistry to suit a variety of compounds.
Injector
This component is used to introduce the sample into the HPLC system. It is typically an autosampler that allows for accurate, repeatable, and reproducible injection of small sample volumes in microliters.
Detector
After the sample exits the column, the detector measures the compounds as they elute. Common detectors include: UV-Vis, fluorescence, and refractive index detectors.
- UV-Vis detectors: Most commonly used due to their ability to detect a wide range of organic compounds as they absorb UV light of different wavelengths.
- Fluorescence detectors: Highly sensitive and selective, used when analytes naturally fluoresce or can be derivatized to fluoresce.
- Refractive Index (RI) detectors are universal detectors used for compounds that do not absorb UV light, like sugars and lipids.
- Mass spectrometers (MS): Coupled with HPLC (HPLC-MS), this detector is highly sensitive and provides structural information about the analytes.
Data Acquisition System
The detector sends signals to a computer system that generates chromatograms, allowing for the visualization and quantification of the analytes.
How HPLC Works
At the heart of HPLC is the principle of partitioning. Sample components interact with the silica embedded in the stationary and mobile phases. These interactions cause differential retention times for each component, leading to separation. The time it takes for a compound to elute from the column is known as its retention time.
- These retention times are influenced by several factors:
- Chemical nature of the analyte: The interaction between the analyte and the column, whether via hydrophobic, van der Waals, or electrostatic forces, determines its retention.
- Mobile phase composition: The polarity, pH, and ionic strength of the mobile phase can significantly influence the separation by altering the analyte-stationary phase interaction.
- Flow rate: The speed at which the mobile phase moves through the column can affect the resolution of the separation.
- Column temperature: The temperature affects the viscosity of the mobile phase, which can influence the flow rate and resolution. It can also impact the analyte-stationary phase interaction.
⦁ Sample Injection:
A small volume of the sample in microliters is injected into the mobile phase stream.
⦁ Separation:
As the mobile phase carries the sample through the column, the interaction between the sample and the column begins. The more a component interacts with the stationary phase, the slower it moves through the column. This results in the separation of the different analytes in the mixture.
⦁ Detection:
As the components exit the column, they pass through the detector, which records their presence. The detector produces a signal, typically a peak on a chromatogram, corresponding to each component.
⦁ Quantification:
The area under each peak in the chromatogram is proportional to the concentration of the component in the sample. This allows for the
Quantification of each substance in the mixture.
Types of HPLC
The separation mechanism in HPLC can be primarily classified into different types based on the phase interactions:
- Reversed-Phase HPLC (RP-HPLC):
- This is the most common form of HPLC, where the stationary phase is nonpolar (C18 columns), and the mobile phase is relatively polar (mixture of buffer and organic solvent such as methanol and acetonitrile). It is particularly useful for separating compounds such as pharmaceuticals ( since most drugs are either weak acids or weak bases), peptides, and small molecules.
- Normal-phase HPLC (NP-HPLC):
- Used for separating highly polar compounds, this method uses a polar stationary phase (e.g., silica gel) and a non-polar mobile phase (e.g., hexane). It is effective for separating isomers or compounds with small differences in polarity.
- Ion-exchange chromatography:
- Ideal for separating charged molecules such as amino acids (ligand-exchange chromatography), proteins, or nucleic acids, and also widely used in purifying water. The stationary phase contains charged groups (e.g., sulfonate or amine), and separation is based on the electrostatic interactions between the analytes and these functional groups.
- Size-exclusion HPLC (SEC): also known as gel permeation chromatography
- SEC is employed for biomolecules (proteins) and polymers, where separation is based on molecular size rather than polarity or charge. Larger molecules elute first, while smaller molecules elute later as they enter the pores of the stationary phase. It is used extensively in protein purification and analysis of large biomolecules like polysaccharides.
- Bio-affinity chromatography:
- It is a specialized technique used in High-Performance Liquid Chromatography (HPLC) for the purification and separation of biomolecules based on specific interactions between a ligand and its target molecule. The stationary phase in bio-affinity chromatography is usually a matrix that is covalently attached to a biologically active ligand, while the mobile phase carries the sample. By controlling the conditions, such as ionic strength, pH, and the presence of competing ligands, bio-affinity chromatography allows for the elution of target molecules with minimal contaminants.
- It is widely used in pharmaceutical research and biotechnology for protein purification and analysis, as well as in clinical diagnostics
- In bio-affinity chromatography, the elution phase is crucial for separating the target biomolecule from the stationary phase.
- Elution can be categorized into two primary types, based on how the bound biomolecule is released from the stationary phase:
- Biospecific Elution: This method involves the use of a specific ligand or molecule that competes directly with the target analyte for binding to the stationary phase. The introduction of a competing ligand (often a free form of the ligand used in the stationary phase) displaces the target molecule from the binding site. This process maintains high specificity and allows for the selective release of the target biomolecule without disturbing other non-target molecules. Biospecific elution is commonly used when a strong, specific interaction exists between the analyte and the ligand, such as antigen-antibody or enzyme-substrate interactions.
- Aspecific Elution: In contrast, aspecific elution occurs when non-selective conditions are used to disrupt the binding between the target biomolecule and the stationary phase. This can be achieved by changing the environmental conditions, such as adjusting the pH, ionic strength, or using denaturing agents. The aim is to weaken or break all types of binding interactions, releasing both the target molecule and other non-specifically bound substances. Aspecific elution is typically used when a broad range of molecules with weak or nonspecific binding need to be eluted simultaneously.
- Elution can be categorized into two primary types, based on how the bound biomolecule is released from the stationary phase:
Key Parameters Affecting HPLC Performance
- Column Efficiency: Measured in terms of theoretical plates (N), column efficiency is critical for the resolution of closely eluting compounds. Higher N values correspond to greater resolution. Theoretical plates (N) should not be<2000.⦁ Resolution: The ability of the column to separate two compounds is quantified by the resolution factor (Rs).
- A resolution greater than 2 is typically considered excellent.
- Retention Factor (k): This is a measure of the time an analyte spends in the stationary phase relative to the time spent in the mobile phase.
- A k-value of 2-5 is usually desired for effective separation.
- Peak Shape: Ideally, chromatographic peaks should be symmetrical. Tailed peaks can indicate problems like column overload, improper mobile phase conditions, or contamination.
- Peak tailing should be less than <2
Applications of HPLC
HPLC finds applications across a broad spectrum of industries and research areas:
- Pharmaceuticals: HPLC is used for drug development, quality control, and validation of pharmaceutical products. It is essential for testing the purity, identity and strength of active ingredients and excipients, as well as for the analysis of complex biological samples.
- Environmental Testing: The technique is employed to detect pollutants in air, water, and soil samples. Analytes like pesticides, heavy metals, and organic pollutants can be identified and quantified using HPLC.
- Food and Beverage: HPLC is utilized in food testing for the identification and quantification of preservatives, additives, and contaminants like pesticides and foodborne toxins.
- Clinical and Forensic Analysis: HPLC is critical in toxicology and drug testing. It is used to detect drug abuse and quantify therapeutic drug levels in
biological fluids like blood and urine - Biotechnology: It is used in the analysis of proteins, nucleic acids, and other biomolecules.
Advantages VS Limitations of HPLC
| Advantages | Limitations |
|---|---|
| High Sensitivity: HPLC can detect low concentrations of substances with great precision. (ppm or even ppt levels). | Cost: HPLC systems can be expensive, both in terms of initial investment and maintenance. |
| Versatility: With various detectors and stationary phases, HPLC can be adapted to analyze a wide range of substances. | Complexity: The setup and maintenance of HPLC systems require specialized knowledge and skills. |
| High Resolution: It can separate complex mixtures into individual components. | Solvent Consumption: HPLC requires a large volume of solvents, which can be a concern for both cost and environmental reasons. |
| Quantification: HPLC provides accurate and reproducible results for the Quantification of analytes. | Time Consumption: While HPLC provides high resolution, it can sometimes be time-consuming, particularly for complex mixtures. |
Conclusion
High-Performance Liquid Chromatography (HPLC) stands as a fundamental technique in modern analytical science, providing exceptional resolution and sensitivity for separating and analyzing complex mixtures. Its broad applications, ranging from pharmaceuticals to environmental monitoring, highlight its indispensable role in both research and industry. As technology advances, particularly with innovations like the integration of HPLC with mass spectrometry (LC-MS), the ability to conduct even more precise and detailed analyses continues to grow, further enhancing its utility and
potential.
- References
- Snyder, L. R., C Kirkland, J. J. (2010). Introduction to Modern Liquid Chromatography (3rd ed.). Wiley-Interscience.
- Guiochon, G., C Schmit, R. (2006). Fundamentals of Preparative and Nonlinear Chromatography. Elsevier.
- Skoog, D. A., Holler, F. J., C Crouch, S. R. (2017). Principles of Instrumental Analysis (7th ed.). Cengage Learning.
- Horvath, C., C Fekete, J. (2002). High-Performance Liquid Chromatography: Principles and Practice. Elsevier.
- A Guide to HPLC and LC-MS Buffer Selection ACE HPLC columns: https:// www.hplc.eu/Downloads/ACE_Guide_BufferSelection.pdf
- Organic modifiers: https://www.hplc.eu/Downloads/AKN0008_OrganicModifiers.pdf
- L. J. Righetti, “Bioaffinity Chromatography: Recent Developments and Applications in Protein Purification,” Journal of Chromatography A, 2015, 1376, 43-58.
- S. K. Singh, “The Principles and Applications of Bioaffinity Chromatography,” Bioseparation, 2017, 22(5), 432-445. B. L. Wang et al., “Elution Strategies in Bioaffinity Chromatography,” Journal of Chromatography B, 2014, 957, 95-102.
- Jandhyala, S. S. R., C Ranjan, P. (2014). Bioaffinity Chromatography for Protein Purification: A Review. Journal of Chromatography B, 962, 15-24.
- Wang, Y., C Reidy, S. L. (2015). Bioaffinity Chromatography: A Review of Recent Advances. Journal of Chromatography A, 1421, 61-71.
- Kau, R. G., C Fenske, D. B. (2009). Principles of Bioaffinity Chromatography. In HPLC of Biological Macromolecules: Recent Developments (pp. 215-246). Wiley- Blackwell


