Abstract
Wet granulation is a critical process in pharmaceutical manufacturing, used to improve the flowability, compressibility, and content uniformity of powders for tablet production. This article explores the principles, mechanisms, equipment, advantages, limitations, and recent advancements in wet granulation technology.
In simple terms, wet granulation involves sticking tiny powder particles together using a liquid binder—like water or a polymer solution—to form larger granules. These resulting granules have better flowability, compress more evenly, and ensure that blend uniformity is attained, which will then reflect on tablets content uniformity.
Wet granulation remains an effective method because of its reliability and versatility. In this article, we’ll break down how it works, the equipment involved, its pros and cons, and how modern advancements are making it even better.
Introduction
The pharmaceutical industry relies on wet granulation for its ability to:
- Enhance powder flowability
- Improve compressibility
- Ensure uniform drug distribution
- Reduce dust generation
- Minimize segregation of components
This article provides a detailed examination of wet granulation, covering its mechanisms, equipment, formulation considerations, and emerging trends.
Early stages of wet granulation technology development employed low-shear mixers, or the mixers/blenders normally used for dry blending such as ribbon mixers. There are several products currently manufactured using these low-shear granulators. However, as process control and efficiency have increased over the years; the industry has embraced high-shear granulators for wet granulation because of its efficient process reproducibility and modern process control capabilities.
The high-shear mixers have also facilitated new technologies, such as one-pot processing, that use the mixer to granulate and then dry in the same unit, the wet mass, using a vacuum, gas stripping/ vacuum, or microwave-assisted vacuum drying. The high-shear one-pot system can be utilized for granulating potent compounds or to produce effervescent granulation. The one-pot system has an advantage especially where an organic solvent is used. It reduces the footprint and minimizes the number of process equipment. The fluid bed processing of powders is a well-established technology.
Previously fluid bed processors were used only as a dryer but now it has become a multiprocessor, where granulation, drying, particle coating, taste masking, and pelletization processes can be performed.
Mechanisms of Wet Granulation
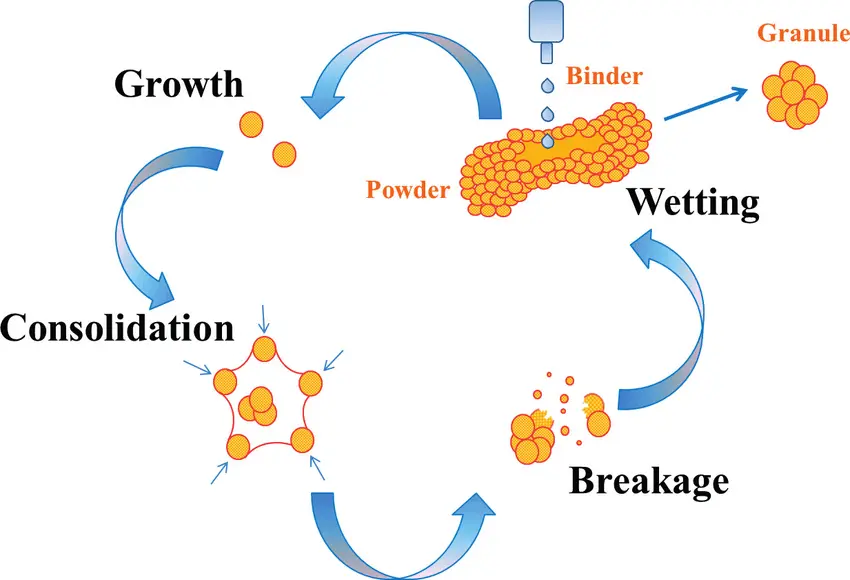
Wet granulation involves several key stages:
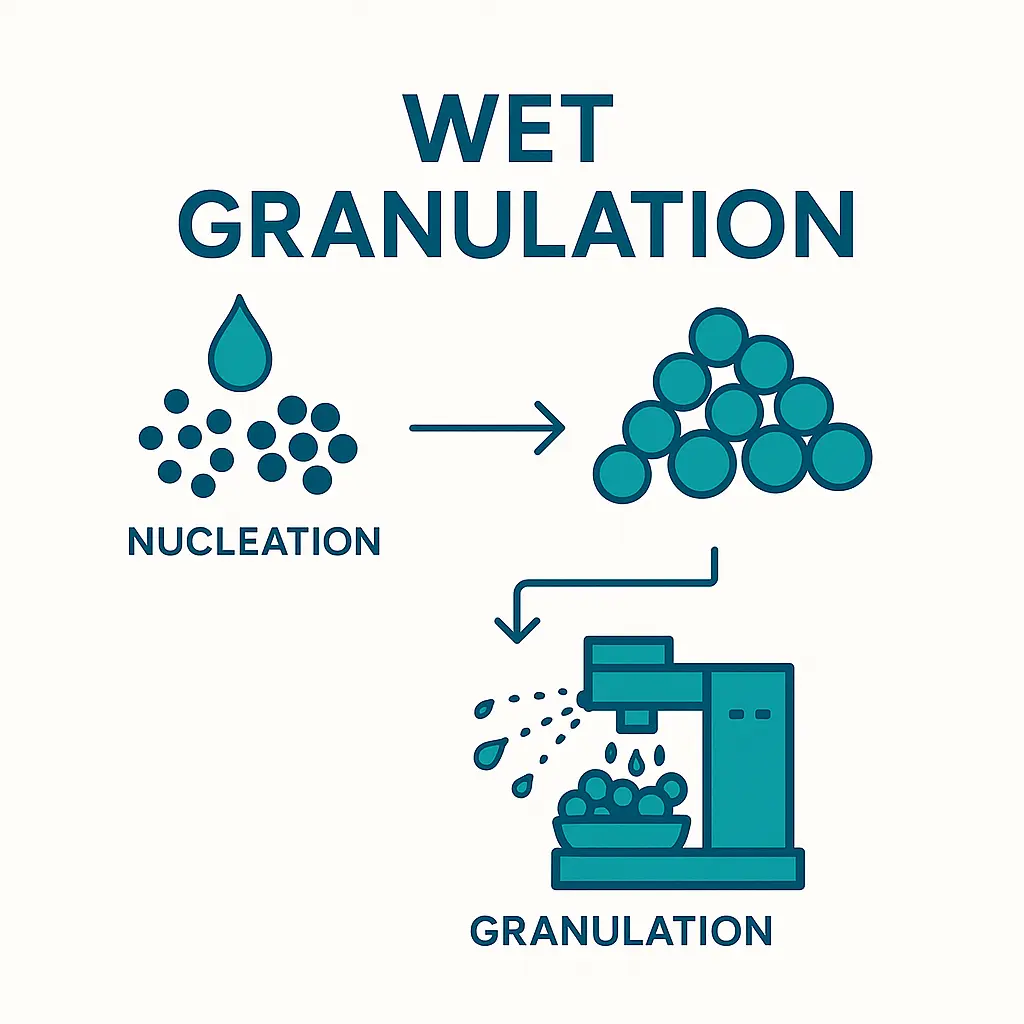
1. Wetting and Nucleation
The process starts with the addition of a liquid binder (e.g., water, ethanol, or polymer solutions) to the powder blend. The liquid bridges between particles initiate nucleation, forming small agglomerates.
2. Consolidation and Growth
As agitation continues, granules grow through coalescence and layering. The liquid binder facilitates particle-particle bonding, while mechanical forces (e.g., mixing, kneading) promote densification.
3. Breakage and Attrition
Excessive shear forces can cause granule breakage, leading to a balance between growth and attrition. Optimal process control ensures uniform granule size distribution.
4. Drying
After granule formation, the wet mass is dried (typically in a fluidized bed dryer or tray dryer) to remove excess moisture and to attain a suitable moisture content, resulting in stable, free-flowing granules.
Why Use Wet Granulation?
1. Better Flow = Better Tablets
Fine powders have bad flow properties, leading to uneven tablet weights. Granules, on the other hand, move smoothly through machines, ensuring each pill has the correct dose (Content uniformity).
2. Stronger, More Uniform Tablets
Granules have better compressibility than loose powder, reducing the risk of weak tablets.
3. Less Dust, Safer Handling
Powders can create airborne dust, which is hazardous for workers. Granulation minimizes this risk.
4. Prevents Ingredient Separation
Some powders separate during handling (like cereal settling in a box). Granulation keeps the mixture uniform.
5. Customizable Drug Release
By choosing different binders, manufacturers can control how fast a tablet dissolves in the body—useful for extended-release medications.
Equipment Used in Wet Granulation
Several types of equipment are employed in wet granulation, each offering unique advantages:
1. High-Shear Mixer Granulators
- Principle: Utilizes high-speed impellers and choppers to mix and granulate powders.
- Advantages: Rapid processing, uniform granule size, reduced liquid requirement.
- Applications: Large-scale production of tablets.
2. Fluidized Bed Granulators
- Principle: Combines granulation and drying in a single unit by suspending particles in an air stream while spraying the binder.
- Advantages: Effective in reducing drying time and performing continuous process
- Applications: Heat-sensitive and moisture-sensitive formulations.
3. Twin-Screw Granulation (TSG)
- Principle: Uses twin-screw extruders for continuous wet granulation.
- Advantages: Scalability, reduced batch-to-batch variability.
- Applications: Continuous manufacturing in modern pharmaceutical plants.
4. Planetary Mixers and Low-Shear Granulators
- Principle: Gentle mixing with a planetary blade or paddle.
- Advantages: Suitable for lab-scale development and shear-sensitive materials.
- Applications: Small-batch R&D formulations.
Formulation Considerations
Successful wet granulation depends on the selection of appropriate excipients and process parameters:
1. Binders
Binders enhance granule strength. Common binders include:
- Natural polymers: Starch, gelatin
- Synthetic polymers: PVP (polyvinylpyrrolidone), HPMC (hydroxypropyl methylcellulose)
- Sugars: Sucrose, glucose
2. Solvent Selection
- Aqueous solvents (water): Economical but may degrade moisture-sensitive drugs.
- Organic solvents (ethanol, isopropanol): Faster drying but require safety precautions.
3. Other Excipients
- Fillers/Diluents: Lactose, microcrystalline cellulose (MCC)
- Disintegrants: Croscarmellose sodium, sodium starch glycolate
- Lubricants: Magnesium stearate (added post-drying)
4. Process Parameters
- Liquid-to-powder ratio: Affects granule density and hardness.
- Mixing time and speed: Influences granule growth and uniformity.
- Drying conditions: Temperature and airflow must be optimized to prevent over-drying or incomplete drying.
Advantages of Wet Granulation
- Improved Powder Flow: Granules flow better than fine powders, ensuring uniform die filling in tablet presses.
- Enhanced Compressibility: Granules withstand compression forces, reducing tablet capping and lamination.
- Uniform Drug Distribution: Minimizes content variability in the final dosage form.
- Dust Reduction: Safer handling compared to fine powders.
- Versatility: Suitable for a wide range of APIs and excipients.
Limitations and Challenges
- Moisture Sensitivity: Not ideal for hydrolytically unstable drugs.
- Longer Processing Time: Includes wet massing, drying, and milling steps.
- Risk of Overgranulation: Excessive liquid can lead to large, hard granules that are difficult to compress.
- Residual Moisture: Can affect drug stability if not properly controlled.
Recent Advances in Wet Granulation
1. Continuous Manufacturing
- Twin-screw granulation (TSG) enables real-time monitoring and quality control.
- Integrated PAT (Process Analytical Technology) tools optimize granule attributes.
2. Quality by Design (QbD) Approach
- Systematic optimization of critical process parameters (CPPs) and critical quality attributes (CQAs).
- DOE (Design of Experiments) models predict granulation outcomes.
3. Novel Binders and Excipients
- Co-processed excipients: Improve functionality (e.g., Ludipress).
- Superdisintegrants: Enhance dissolution of poorly soluble drugs.
4. Green Granulation Technologies
- Solvent-free granulation: Uses meltable binders (e.g., PEG-based).
- Microwave-assisted drying: Reduces energy consumption.
Conclusion
Wet granulation remains a critical step of pharmaceutical manufacturing due to its ability to improve powder properties and ensure product quality. By optimizing formulation and process parameters, manufacturers can achieve high-quality granules for robust tablet production.
References
- Handbook of Pharmaceutical Granulation
- Journal of Pharmaceutics, AAPS PharmSciTech
- Journal of Pharmaceutical Sciences.


