Stability studies are mandatory; it is vital. Over time, it has demonstrated the significance of stability testing and its critical role in ensuring a safe drug for patients. In this article, we will briefly cover the stability testing protocol, its guidelines, and highlight a case study.
Stability studies are a fundamental component of pharmaceutical development and regulatory compliance. These studies aim to assess the effects of various environmental factors, including temperature, humidity, light, and pH, on the quality, safety, and efficacy of a drug product over time. This work provides a comprehensive discussion on the principles and applications of stability studies.
Types of stability studies
There are three types of stability studies; let’s discuss them briefly:
1-forced stability testing
Stress testing exposes the drug to extreme conditions to identify degradation pathways and assess the molecule’s intrinsic stability. Here, in the stress conditions used in forced stability testing.


Showing in this table, how to apply this test to the drug?
| Application | Key parameter testing |
| Required to validate stability-indicating analytical methods and identify potential degradation products as per ICH Q1A and Q1B. | Testing evaluates the susceptibility of the drug substance to hydrolysis |
2. Long-Term Stability Testing
In this test, it depends on Real-time stability testing, which involves storing drug samples under recommended conditions for extended periods and assessing them at predetermined intervals. This is the most dependable method for determining actual shelf life. The drug is exposed to standard conditions, which are discussed below.
The test duration, application procedures, and practical significance are presented in the table and discussed in detail below.
| Test duration | Application | Key parameter testing |
| up to 24 or 36 months analysis at 0, 3, 6, 9, 12, 18, and 24 months | Establishing official shelf life | AssayDegradation productsAppearanceCointainer closure |
You can check more about degradation products in Navigating the Critical Complexities of Impurities and Degradation Products in Drug Development Part (2)
3- Accelerated stability testing
It is performed to predict the long-term stability of a drug product in a shorter period. By exposing the drug to elevated stress conditions (typically higher temperatures and humidity), according to ICH Q1A(R2), accelerated testing should be conducted at 40°C ± 2°C and 75% RH ± 5% RH
showing in the table, the test duration, and its application:
| Test duration | Application |
| 6 months | The prediction of impurities can be assessed in a shorter time |
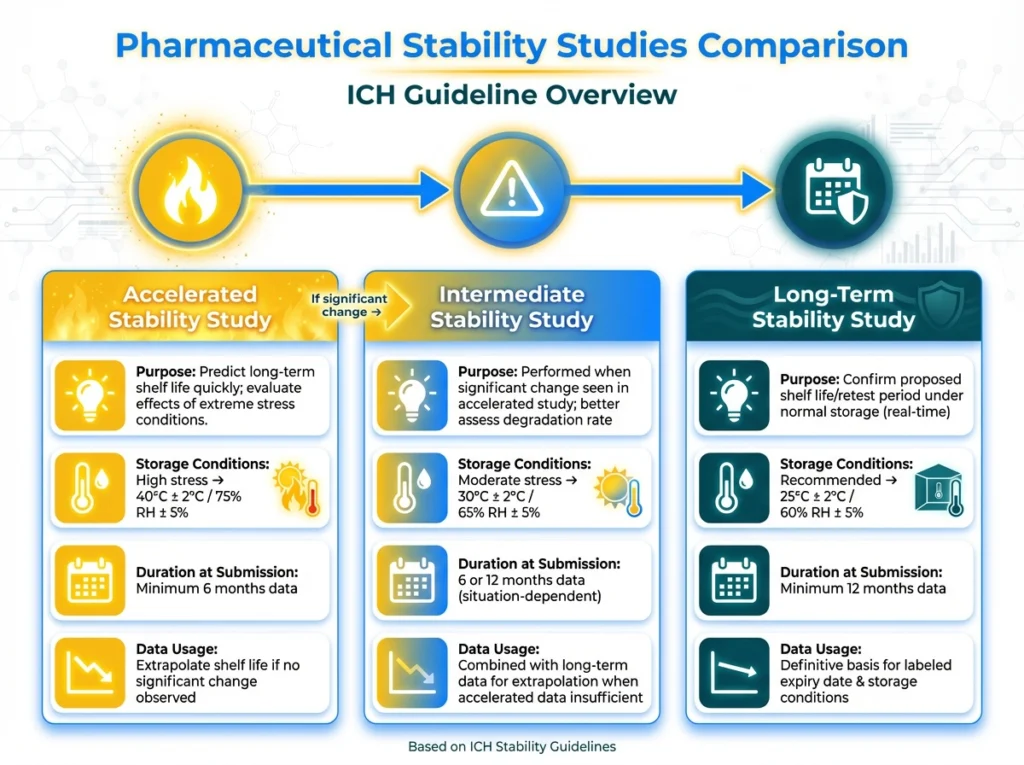
Another test could be done in some situations:
- intermediate stability testing
- Sometimes, intermediate testing is needed, which is done at 30°C ± 2°C and 65% RH ± 5% RH for 6 months
- photostability testing
- Another test was done according to the ICH, at least for one batch
After that, we are going to review in this section the regulatory frameworks and international guidelines that govern the design, execution, and evaluation of stability studies. Emphasis is placed on compliance with established standards, such as the International Council for Harmonisation (ICH) guidelines
Regulatory Compliance and ICH Guideline:
The International Council for Harmonisation (ICH) has established a set of global standards for stability testing that are widely recognized by regulatory authorities worldwide, which include:
- ICH Q1A (R2): Stability Testing of New Drug Substances and Products
- ICH Q1B: Photostability Testing
- ICH Q1C: Testing of New Dosage Forms
- ICH Q1D: Bracketing and Matrixing Designs
- ICH Q1E: Evaluation of Stability Data
- ICH Q1F: Stability Data Requirements for Global Submission (now withdrawn but still referenced by some agencies)
- ICH Q1EWG: Stability testing for drug substances and drug products.
What are these guidelines used for?
For any professional study of stability, those guidelines should be applied that provide:
- Study Design: Defines the overall structure and protocols for stability studies.
- Testing Parameters: Specifies the attributes to be tested, such as potency, purity, and dissolution rate.
- Storage Conditions: Details the environmental conditions (temperature, humidity, and light) under which the products are stored during the study.
- Documentation Requirements: Outlines the necessary documentation for demonstrating compliance with regulatory standards.
Adherence to these international regulatory frameworks ensures that stability studies are scientifically sound and globally acceptable. Following the establishment of these requirements, a critical next step is the careful selection of the batches to be tested. So, selecting the batch that is going to be tested is critical for maximum efficiency of the study.
Selecting Suitable Batches
According to ICH, the batch chosen should be:
- Manufactured to a minimum of pilot scale
- Manufactured with the final formulation and packaging
- Subjected to a validated analytical method
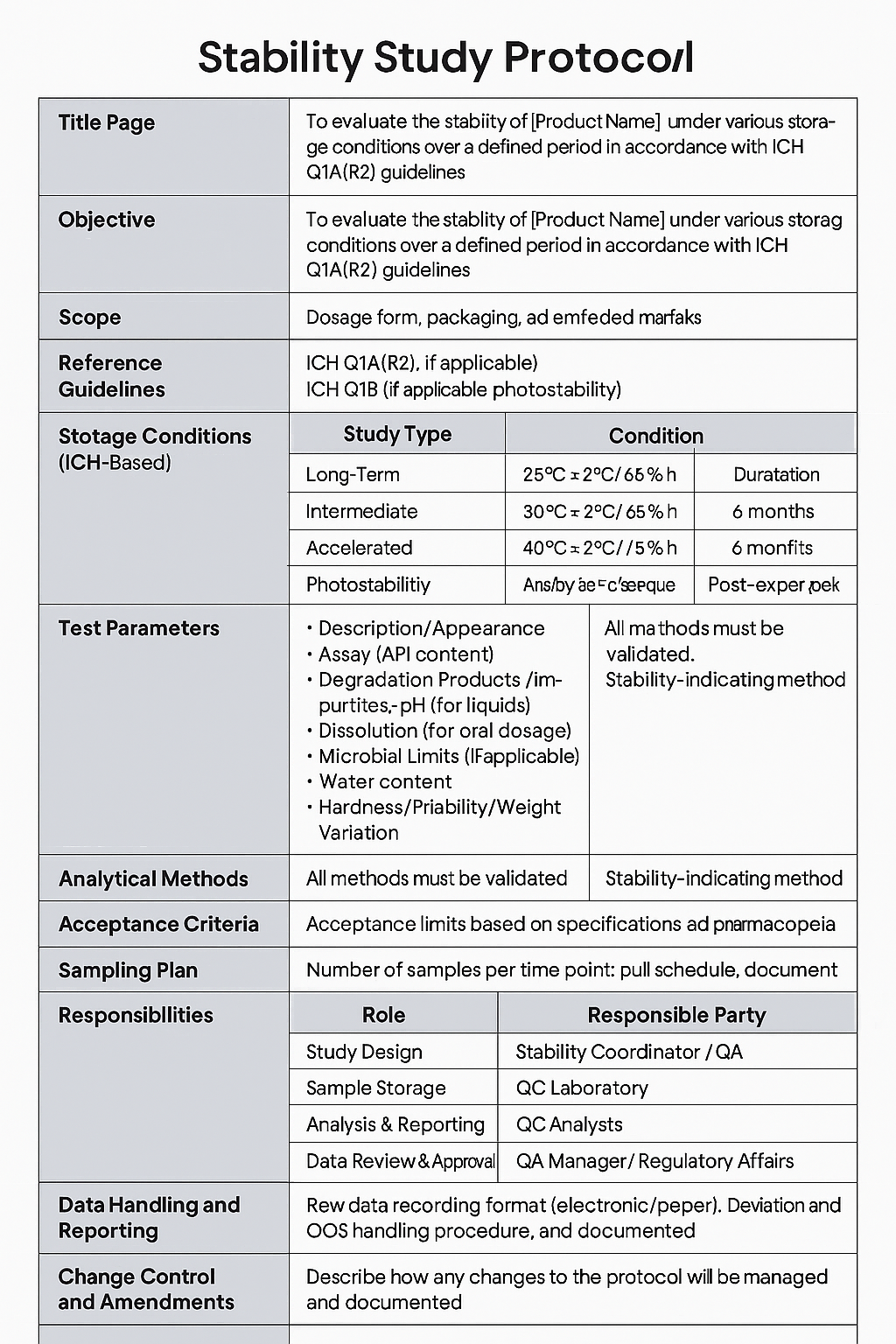
Following batch selection, a detailed stability study protocol must be developed according to the applicable guidelines. This protocol serves as the blueprint for the entire study, outlining the objectives, selected batches, storage conditions, testing intervals, analytical methods, acceptance criteria, and data reporting formats.
How to make a protocol?
Documentation is essential in a stability study, so we are going to discuss the steps needed for documenting your data. To ensure the reproducibility of the protocol, it should follow the outlines of how the study will be conducted. It ensures regulatory compliance with ICH as ICH Q1A(R2), WHO, EMA, or FDA standards.
- Title of page which includes:
- Title page
- Product code
- Batch number
- Dosage form
- Api name
- Protocol number and name
- Date of issue
- Authorized signature
- Describe the Objective.
- Describe the scope of the study
- Reference Guidelines
- product information
- Storage Conditions (ICH-Based)
- Test Parameters
- Analytical Methods
- Acceptance Criteria
- Sampling Plan
- Responsibilities
- Data Handling and Reporting
- Stability commitment
- Annexes
showing an example of protocol:
Stability Profile of Erlotinib: Comparing Marketed Tablets with Extemporaneously Oral Suspensions
First, erlotinib is an orally active epidermal growth factor receptor (EGFR) tyrosine kinase inhibitor used in the treatment of non-small cell lung cancer (NSCLC) and pancreatic cancer. As a crucial anticancer drug, its stability is paramount for patient safety and therapeutic efficacy. Many analytical studies and formulation development have focused on understanding Erlotinib’s degradation pathways and ensuring its stability in various conditions and formulations.
Second, the chemical structure of Erlotinib is a quinazoline derivative with specific functional groups like ethynyl, methoxy, and ethoxy, making it susceptible to degradation under certain environmental stresses. For instance, the quinazoline ring and amine ketone groups can be points of vulnerability.
- The Erlotinib’s Degradation Pathways(Forced Degradation Studies):
This section showed forced degradation studies were conducted to systematically evaluate the chemical behavior of Erlotinib under various stress conditions, acting as individual “cases” of how the drug behaves under stress. Taking into consideration that this testing is independent of the dosage form.
Stress Conditions and Observed Degradation:
- Acidic Hydrolysis: Erlotinib is known to be susceptible to acidic hydrolysis.
- Studies have shown degradation under strong acidic conditions as 1N HCl. This is clinically relevant, as gastric pH can affect oral absorption.
- Alkaline Hydrolysis: Degradation also occurs under basic conditions as 1N NaOH.
- This highlights its sensitivity to pH changes.
- Photolytic Degradation: Erlotinib shows susceptibility to light degradation, especially in solution form UV light, fluorescent light exposure.
- This underscores the need for light-protective packaging.
- Oxidation: While some studies suggest stability under typical oxidative stress, others indicate mild oxidation and N-oxide formation under specific photolytic conditions.
- Thermal Degradation: Erlotinib generally shows good stability under dry heat conditions.
Secondly, in this section, we will discuss how a long-term stability study is applied. Taking into consideration that this test differs according to the dosage form of the drug.
- Long-term Stability:
For the Case of Erlotinib’s Formulation Stability Oral Tablets:
Long-term stability studies of marketed Erlotinib oral tablets, packaged in aluminum–aluminum (Al–Al) blister packs, have demonstrated acceptable stability over approximately 2–3 years when stored under recommended conditions (room temperature, protected from light and moisture). Showing the main role of the package.
The Al–Al blister packaging plays a critical role in maintaining Erlotinib stability by providing an effective barrier against light, oxygen, and moisture. Given the drug’s susceptibility to photolytic and oxidative degradation, to maintain consistent product quality during storage and distribution.
For extemporaneously Prepared Oral Suspensions:
Although Erlotinib is commercially available as tablets, some patient populations that complain of dysphagia or pediatric patients receiving off-label therapy require liquid dosage forms. Consequently, extemporaneous oral suspensions prepared from crushed tablets are sometimes necessary in clinical practice.
Due to its liquid form, it will face more stability challenges:
- Solubility: Erlotinib’s decreased solubility at pH above 5 is a significant challenge when preparing suspensions, as pH changes can lead to precipitation or poor dissolution.
- Excipient Compatibility: The choice of suspending vehicle, like Ora-Plus, Ora-Sweet, is critical.
- Studies have shown that Erlotinib suspensions in certain vehicles can retain potency for specific durations. These findings emphasize that excipient compatibility is a major determinant of suspension performance.
- Physical Stability: Viscosity changes, sedimentation, or “pudding-like” texture can occur, impacting dose accuracy and patient acceptance.
- Poor physical stability also increased the risk of inconsistent dosing if adequate shaking was not performed before administration.
- Chemical Stability: The API can degrade more rapidly in solution than in solid form due to increased molecular mobility.
Sampling was conducted at predefined intervals and analyzed using a stability-indicating high-performance liquid chromatography (HPLC) method, validated according to ICH Q2(R1) for specificity, linearity, accuracy, and precision.
Conclusion
The stability case study of Erlotinib highlights the fundamental differences between the marketed oral tablet formulation and the extemporaneously prepared oral suspension case study of Erlotinib’s stability provides a valuable illustration of the complexities in maintaining the quality of potent anticancer drugs from manufacturing through to patient administration, highlighting the critical measures required to preserve maximum efficacy during storage. As shown in the figure below, erlotinib tablets offer long-term stability and reliable dosing, while extemporaneous suspensions are less stable and require careful handling for special patients.

References
Lab Manager (2022). Stability Testing of Pharmaceuticals: Procedures and Best Practices.
Retrieved from https://www.labmanager.com/stability-testing-of-pharmaceuticals-procedures-and-best-practices-33944
Egyptian Drug Authority (2022). Guidelines on Stability Testing of Finished Pharmaceutical Products and Active Drug Substance.
Available at https://www.edaegypt.gov.eg/media/uaghtum1/القواعد-المرجعية-للإدارة-العامة-للثبات.pdf
International Council for Harmonisation (ICH). Quality Guidelines: Stability Testing (ICH Q1A–Q1F).
Accessed via https://www.ich.org/page/quality-guidelines
Pharma Guide (2024). Stability Study Protocol Format and Best Practices.
Available at https://www.m-pharmaguide.com/2024/07/stability-study-protocol.html
Cohen, J., Lee, C., Markham, R., Szerwo, J., Roska, M., & Bubalo, J. (2022).
Medication Use Process and Assessment of Extemporaneous Compounding and Alternative Routes of Administration of Oral Oncology Drugs: Guidance for Clinical and Oncology Pharmacists.
Journal of the American College of Clinical Pharmacy, Published online: August 27, 2022.
https://doi.org/10.1002/jac5.1698
Mahajan, A.A., Miniyar, P.B., Patil, A.S., Tiwari, R.N., et al. (2015).
Separation, Identification, and Characterization of Degradation Products of Erlotinib Hydrochloride under ICH-Recommended Stress Conditions by LC and LC-MS/TOF.
Journal of Liquid Chromatography & Related Technologies, 38(5), 629–639.
https://doi.org/10.1080/10826076.2014.936610
Nguyen, D., Secrétan, P.H., Cotteret, C., Jacques-Gustave, E., Greco, C., Bodemer, C., Schlatter, J., & Cisternino, S.
Stability and Formulation of Erlotinib in Skin Creams.
In: Journal [Editor: Dániel Nemes, Ildikó Bácskay] — [Journal details and DOI to be inserted if available].