Similarity Factor (F1, F2) Calculator
Effortlessly compare test and reference dissolution profiles with our smart f₁/f₂ calculator. Visualize data in interactive charts, export results to Excel, and download publication-ready graphs, all in one click. Perfect for QC, R&D, and formulation scientists seeking ease of access, accuracy, and clarity .
Similarity Factor (f₁ / f₂) Calculator
Enter comma-separated time points and % release values. Click “Calculate” to get f₁ / f₂ and view the dissolution profile chart.
What Is Similarity factor?
- The similarity factor (f₂) and difference factor (f₁) are statistical tools recommended by the FDA and EMA to compare the dissolution profiles of two products: a test formulation and a reference (usually innovator) product.
- They help assess bioequivalence, batch consistency, and formulation changes during product development and scale‑up.
- It is a critical quality control (QC) and development tool to evaluate drug product performance, bioavailability, and consistency.
Scientific Basis for Calculation
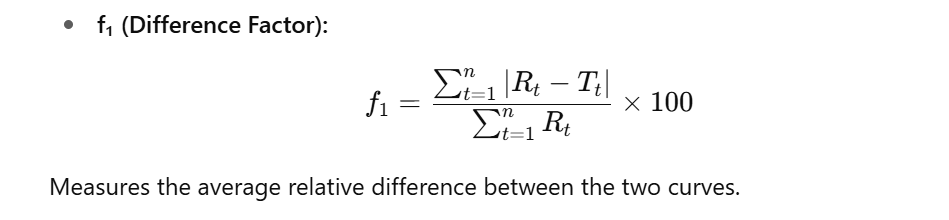
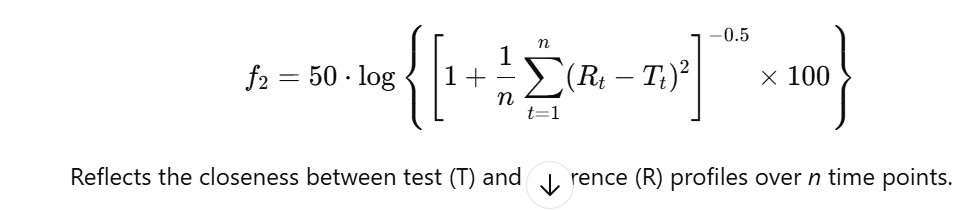
Components Explained:
| Field Name on Calculator | Scientific Term | Description |
|---|---|---|
| Reference % release | Rₜ | percentage dissolved at time point t for the reference product |
| Test % Release | Tₜ | percentage dissolved at the same time point t for the test product |
| Automatic calculation integrated | n | total number of sampling points (usually 3–6, excluding the zero point) |
Scientific Notes & Tips for This Calculator
- Acceptance Criteria
- f₂ ≥ 50 → profiles are considered similar
- f₁ ≤ 15 → profiles have low difference
- These criteria apply when:
- Number of time points: typically 3–6 (excluding zero)
- ≥ 12 units tested per product
- Variability (CV%) ≤ 20% at early points and ≤ 10% at other points
- Use the same time points for test & reference profiles.
- Avoid too many or too few time points (ideal: 4–5 after 85% dissolution).
- Exclude zero time point from calculation.
- Graph the curves to visualize deviations.
- Why It Matters: Using f₁ / f₂ ensures:
- Regulatory compliance (FDA, EMA)
- Consistent in vitro performance
- Reduced risk of batch failure
- Our calculator simplifies this by:
- ✔ Automating the formula
- ✔ Providing clear pass/fail flags
- ✔ Generating charts and Excel reports for documentation