1. Introduction: Why Sustainability Matters in Pharma

The pharmaceutical industry has long been associated with life-saving innovation, but it’s also becoming increasingly linked to an uncomfortable truth: its environmental footprint is far larger than many realize. From solvent-laden synthesis pathways to single-use plastic-heavy drug delivery systems and chemically persistent excipients, the pharma pipeline, while clinically transformative, has historically been environmentally intensive.
A study by The University of York’s Global Monitoring of Pharmaceuticals Project (2022) found that over 43% of sampled global rivers were contaminated with pharmaceutical residues, including antibiotics, antidepressants, and painkillers. These pollutants don’t just disappear; they accumulate in ecosystems, disturb microbial balance, induce antimicrobial resistance, and even affect reproductive systems in aquatic wildlife.
But this environmental toll doesn’t stop at APIs. The manufacturing, packaging, and delivery mechanisms themselves contribute to:
- High carbon emissions (energy-intensive synthesis and drying methods)
- Excessive plastic and aluminum waste (especially from blister packs, injectables, and inhalers)
- Post-consumer contamination, with patients discarding medicines improperly or flushing unused drugs into wastewater systems
1.1 The Green Paradox
Ironically, while the pharmaceutical industry exists to heal humans, its operations often contribute to environmental conditions that harm broader health, air pollution, hormone disruption in water, antibiotic resistance, and climate change.
In this light, sustainability is no longer a CSR initiative; it’s a scientific, ethical, and operational imperative.
1.2 The Shift Toward Eco-Conscious Drug Design
Over the last decade, sustainability in pharma has evolved from generic energy-saving goals to something much more nuanced and strategic: green-by-design drug delivery systems. That means rethinking every part of a medicine’s lifecycle, from excipient sourcing and processing solvents to how a tablet degrades in the gut, and even how its packaging composts in landfills.
This new paradigm demands a move toward:
- Biodegradable polymers in tablets, capsules, patches, and injectables
- Renewable and biocompatible excipients that don’t bioaccumulate or persist post-excretion
- Green chemistry principles in formulation and manufacturing
- Sustainable packaging that protects drug integrity without harming the planet
1.3 Why Drug Delivery Systems (DDS) Are the Frontline
While green synthesis has received attention in recent years, drug delivery systems themselves represent the most consumer-visible and environmentally persistent aspect of pharma. DDSs are in direct contact with the patient and environment and often account for:
- The highest surface contact volume with biological and ecological systems
- The largest proportion of post-consumer pharmaceutical waste
- The slowest degradation profiles, especially in the case of long-acting devices, transdermal patches, and injectables
Thus, making DDS eco-friendly is not just impactful, it’s the most actionable and scalable starting point for greener pharma.
1.4 The Bigger Picture: Global Pressures & Regulatory Wake-Up
Governments, regulators, and even investors are now demanding measurable climate and sustainability action from pharmaceutical manufacturers. For example:
- The European Green Deal and EU Pharmaceutical Strategy call for an eco-design approach to drug development.
- The FDA’s Center for Drug Evaluation and Research (CDER) is exploring sustainability data in CMC sections.
- ESG-focused investment groups are scrutinizing drug developers based on their supply chain, environmental waste management, and product lifecycle planning.
Even public health bodies like the World Health Organization (WHO) have labeled pharmaceutical pollution as an emerging global threat, putting additional pressure on manufacturers to prove their ecological responsibility.
1.5 From Innovation to Obligation
In the coming years, companies that proactively embed sustainability into their formulation science will gain regulatory goodwill, operational resilience, and brand trust. Those that don’t may face rising scrutiny, higher costs, and market rejection.
“In the 20th century, we designed drugs to act precisely on the human body. In the 21st century, we must also design them to be gentle on the Earth.”
Ultimately, the movement toward eco-friendly, biodegradable DDS isn’t optional, it’s a matter of futureproofing pharmaceutical science itself.
“We are not only curing patients, we’re also shaping the planet’s health.” A common sentiment is gaining traction in modern formulation labs.
2. The Environmental Toll of Traditional Drug Delivery Systems (DDS)

It’s an uncomfortable truth we don’t often talk about in our labs or boardrooms, our industry, built to save lives, is silently contributing to the environmental crisis.
Drug delivery systems (DDS), the very vehicles we rely on to deliver life-changing therapies, are at the heart of the problem. Though clinically effective, they’ve traditionally been engineered with only two priorities: efficacy and stability. Environmental impact? An afterthought, if considered at all.
But as our rivers darken, soil profiles change, and animal populations suffer mysterious declines, the pharmaceutical world is being forced to reckon with a fact long ignored:
What we design to enter the body doesn’t just disappear after it leaves.
2.1 Pharmaceutical Residues in Ecosystems
Let’s start where most patients assume the medicine journey ends, the toilet.
After ingestion or injection, a significant portion of many drugs (especially poorly absorbed ones or extended-release formulations) exits the body unmetabolized. These active compounds then flow into municipal wastewater systems that were never designed to filter out pharmaceuticals.
Wastewater treatment plants, even in high-income countries, typically remove only 30–70% of pharmaceutical contaminants. That means millions of tons of APIs and excipients seep into rivers, lakes, and oceans every year.
What Happens Then?
These seemingly minuscule residues cause large-scale, long-term biological disruption.
- Antibiotics in rivers contribute to the rise of antibiotic-resistant genes in aquatic microorganisms. These resistance traits can leap from species to species, ultimately reaching humans and making critical antibiotics obsolete.
- Antidepressants and anxiolytics accumulate in the brains of fish, altering behavior, feeding, and reproduction patterns. A 2021 study found that perch exposed to SSRIs became hyperactive, careless, and more likely to die from predation.
- Hormonal drugs, especially ethinyl-estradiol (from contraceptive pills), have feminized male fish populations, disrupted frog reproduction, and thrown delicate aquatic hormone cycles into chaos.
- NSAIDs like diclofenac, widely used for pain relief in livestock, leach into the environment via carcass disposal. In India, this single drug has been linked to a catastrophic 95% collapse in the vulture population, a crisis so severe it destabilized entire rural ecosystems dependent on scavengers.
All of this stems from the DDS systems we proudly label “sustained-release” or “patient-friendly.” Effective? Yes. Sustainable? Not even close.
The Silent Accumulation
What’s most dangerous isn’t the immediate toxicity, it’s the persistence.
Unlike naturally occurring compounds that degrade, most pharmaceutical actives and DDS excipients are engineered to resist breakdown, to remain stable in blood, withstand gut enzymes, survive high-speed manufacturing, and retain potency on pharmacy shelves. But when they enter the environment, this same stability becomes a curse.
They bioaccumulate in marine food chains. They disrupt microbial balance in soil. They linger in drinking water. And as new APIs, polymer coatings, and delivery vehicles get ever more complex, our ability to control their afterlife becomes even more fragile.
2.2 Non-Degradable Packaging & Devices
Let’s zoom out from the microscopic and look at the macro-scale issue waste.
Blister packs. Pre-filled syringes. Transdermal patches. Inhalers. Implants. What do they have in common? They’re all made with multi-material composites designed for product integrity and patient usability, but not for environmental responsibility.
The Numbers Are Stark:
- Over 190 billion pharmaceutical blister packs are produced globally each year
- More than 8 million inhalers are discarded annually in the UK alone, releasing potent greenhouse gases
- Single-use syringes, often made of polypropylene and PVC, take over 400 years to degrade
- Multi-layer drug device components (aluminum + plastic + adhesives) are nearly impossible to recycle
Why We Can’t Recycle Them Easily
Pharmaceutical packaging often contains:
- Foil for barrier protection
- Plastics like PET, HDPE, PVC, or PVDC for structure and sealing
- Inks, adhesives, and coatings for branding and tamper-proofing
- Medical-grade rubber or silicone for closures
These materials can’t be separated in standard recycling streams. Most municipalities treat them as non-recyclable hazardous waste. So they end up in landfills or worse, incinerators, releasing microplastics, dioxins, and greenhouse gases.
The Ethical Dilemma
As scientists and professionals in pharma, we often marvel at the engineering brilliance behind an orally disintegrating film or a pH-responsive capsule. But how often do we ask: “What happens to this after its job is done?”
A delivery system’s responsibility shouldn’t end at bioavailability; it must extend to biodegradability.
Unseen Consequences
While the pharmaceutical industry still contributes less total waste than sectors like oil or fast fashion, the uniqueness of our waste—its biologically active, persistent, and sometimes toxic nature, makes its impact disproportionately dangerous.
Even if we represent only 3% of global plastic use, our share of irreversibly persistent materials is much higher.
And the worst part?
Unlike visible oil spills or plastic straws in turtle noses, pharmaceutical pollution is invisible, cumulative, and insidious. It won’t make headlines, until it’s too late.
Time for Accountability
We can no longer hide behind the excuse that “health comes first.” Health must include environmental health. The drug delivery systems we design today must not solve one problem only to cause another.
It’s time we ask ourselves, in every formulation discussion: “Is this system not only safe and effective, but also responsible?”

3. Biodegradable Materials: The Building Blocks of Green Drug Delivery Systems (DDS)
As scientists and formulation professionals, we know that the delivery system is often as critical as the drug itself. But here’s a new layer of responsibility: What happens after the delivery is complete?
Traditional DDS materials, while brilliant at protecting APIs, modulating release, or targeting absorption sites, often persist in the environment or in biological systems far beyond their therapeutic window. That’s where biodegradable materials come in, offering a future where efficacy and environmental safety are no longer at odds.
Biodegradable DDS are designed to fulfill their therapeutic mission and then gently disappear, degrading into biologically harmless byproducts like CO₂, water, and biomass. These materials aren’t just smart, they’re responsible.
Let’s look at the two key classes that are leading this revolution.
3.1 Natural Polymers: Sustainability from Nature Itself
Natural polymers are sourced from renewable biological origins, plants, sea creatures, or fermentation processes, and have been used for decades in both food and medicine. What’s changed recently is how intelligently we’re repurposing them for biodegradable DDS.
Chitosan
Sourced from crustacean shells, chitosan is a gift from the ocean. Known for its mucoadhesive properties, it clings to mucosal surfaces like intestinal walls or nasal cavities, making it a powerful tool for extended contact and absorption. What’s more, it’s inherently antimicrobial, which can reduce preservative needs in formulations.
Chitosan nanoparticles have been explored for insulin nasal delivery, reducing the need for injections, a human-centric and green innovation.
Alginate
Derived from brown algae and kelp, alginate forms gentle hydrogels in the presence of calcium ions. This ionic gelation allows for controlled drug encapsulation and release, especially useful for oral or injectable formulations. And it’s entirely digestible.
Oral alginate beads have shown promise in protecting probiotics through the stomach’s acidic environment, ensuring delivery to the intestine, while fully breaking down in the GI tract.
Cellulose Derivates (e.g., HPMC, CMC)
Cellulose is the world’s most abundant biopolymer, and its derivatives like hydroxypropyl methylcellulose (HPMC) are cornerstones of green formulation. They’re chemically stable, customizable in viscosity and solubility, and already well accepted by regulators.
HPMC is the backbone of most vegetarian capsules today, a sustainable replacement for gelatin.
Gelatin & Collagen
These animal-derived proteins are still staples in pharma, especially for soft-gel capsules, dissolvable films, and wound healing matrices. They’re biocompatible, biodegradable, and processed efficiently by the body, although there are ethical and allergy-related concerns for some patient populations.
Biodegradable wound dressings made from collagen not only reduce medical waste, they accelerate tissue regeneration naturally.
3.2 Synthetic Biodegradable Polymers: Precision Meets Purpose
While natural polymers offer sustainability and safety, synthetic biodegradable polymers bring performance customization. These materials are engineered for controlled degradation, allowing scientists to fine-tune release rates, mechanical strength, and tissue interaction.
They degrade through hydrolysis or enzymatic action, and their byproducts are easily metabolized or excreted. Let’s explore a few front-runners.
Polylactic Acid (PLA)
Made from fermented plant starches like corn or sugarcane, PLA is a well-known material in resorbable sutures and orthopedic pins. It breaks down into lactic acid, a natural metabolic byproduct. PLA’s strength, biodegradability, and non-toxicity make it ideal for short-term implants and 3D-printed drug matrices.
Polyglycolic Acid (PGA)
This polymer degrades faster than PLA, thanks to its high hydrophilicity. It’s often blended with PLA to balance durability and degradation, most notably in surgical sutures or burst-release injectable formulations. In DDS, PGA’s quick breakdown supports fast-acting, high-bioavailability systems without the need for excipient washout protocols.
Poly(lactic-co-glycolic acid) (PLGA)
Perhaps the most widely studied and FDA-approved biodegradable polymer, PLGA combines the best of PLA and PGA. By adjusting the lactic : glycolic ratio, scientists can control the degradation from a few days to several months. PLGA microspheres have revolutionized long-acting injectables like Lupron Depot (leuprolide acetate), reducing patient burden and clinical visits while leaving no environmental trace.
Polycaprolactone (PCL)
PCL degrades much slower over months to years, making it suitable for long-term implants, scaffolds, and sustained DDS. Its mechanical flexibility and biocompatibility are ideal for tissue engineering and ocular delivery.
Why This Material Revolution Matters
Unlike conventional materials, biodegradable DDS don’t leave behind microplastics, endocrine disruptors, or polymeric residues that haunt soil and water systems for decades. They align with:
- Circular economy principles in pharma
- Green chemistry goals by eliminating persistent pollutants
- Patient safety, as they reduce excipient load and improve tolerability
- Global regulations, which are increasingly pushing for sustainability reporting in dossiers
Most importantly, they shift the narrative from designing drugs only for biological performance to designing them with life-cycle consciousness.
Nature Meets Engineering: A Formulator’s Dream
This is where science gets poetic. We’re not just designing delivery systems anymore, we’re composing degradable symphonies of molecules that deliver healing and then dissolve into harmony with the body and the Earth.
4. Designing Effective Biodegradable DDS: Beyond the Buzzwords
Creating a biodegradable drug delivery system (DDS) is not as simple as replacing one polymer with another. Sustainability in formulation is not a patch job, it’s a systems-level redesign. We’re not just talking about material swaps. We’re talking about reimagining performance, patient experience, and environmental fate, together. At the heart of this shift is the need to intelligently balance four competing factors:
- Biocompatibility
- Controlled drug release
- Formulation stability
- Patient usability and compliance
Designing such a system takes nuance. It’s less like building a machine and more like composing a piece of music, where everything must be in harmony, or the whole thing fails.
4.1 Surface Engineering: Precision at the Interface
In DDS, surfaces matter. Not just what’s inside, but how the material interfaces with biology, from the moment it contacts mucosa, circulates in the bloodstream, or enters a tumor microenvironment. Surface engineering is the art and science of tailoring the outermost layers of particles, implants, and matrices to guide behavior like:
- Where the DDS goes
- How long it stays
- What cells it interacts with
- Whether the immune system ignores or attacks it
Targeting Through Surface Functionalization
One powerful example is PLGA nanoparticles. On their own, they’re biodegradable, biocompatible, and reasonably efficient. But coat them with polyethylene glycol (PEG), and you suddenly give them a stealth shield, invisible to macrophages, extending circulation time. Add RGD peptides, and you can make these same particles home in on tumor cells expressing integrin receptors. This is not just targeting. It’s biological precision tuning.
Think of the surface as a handshake between science and the human body. You’re not just giving a drug, you’re asking biology to accept it gracefully.
Reducing Immunogenicity
Surface modifications also help prevent unwanted immune responses. For instance, cationic (positively charged) particles tend to trigger inflammation. But slight tweaks, such as introducing neutral polymers or hydrophilic coatings, can reduce that reaction while maintaining cellular uptake. Surface engineering is where efficacy meets safety. And it’s a key to green DDS because it allows for lower doses, less systemic exposure, and fewer formulation burdens downstream.
4.2 Stimuli-Responsive Biodegradation: Smarter Than Just “Slow Release”
The next generation of biodegradable DDS isn’t just breaking down over time, it’s thinking. Formulators are now designing systems that respond to biological cues, releasing their payload only when and where it’s needed. This approach reduces:
- Systemic toxicity
- Off-target exposure
- Dose frequency
And perhaps most importantly, it aligns beautifully with eco-design, because it minimizes drug waste and degradation byproducts.
pH-Sensitive Carriers
Take chitosan–tripolyphosphate nanoparticles. In the bloodstream, they’re stable. But expose them to the acidic microenvironment of a tumor (pH ~6.5) and they begin to erode, releasing drug directly at the site of interest. It’s targeted, efficient, and green.
Thermo-Responsive Hydrogels
PLGA–PEG–PLGA triblock copolymers are equally smart. At room temperature, they’re injectable liquids. But inside the body (37°C), they transition to gels, forming a depot that slowly biodegrades while releasing drug. No surgery, no retrieval, no non-degradable waste.
Enzyme-Triggered Degradation
Researchers are also developing systems that degrade in the presence of specific enzymes, like MMPs (matrix metalloproteinases), which are overexpressed in inflamed or cancerous tissues. This allows DDS to selectively activate only where pathology exists.
This is not science fiction, it’s happening right now in oncology, ophthalmology, and even gene therapy pipelines.
Stimuli-responsive DDS are the bridge between pharmacology and physiology, allowing delivery systems to behave like they understand the human body. That intelligence is key to making green systems viable, not just in theory, but in clinical reality.
4.3 Green Processing Techniques: The Invisible Footprint
It’s easy to focus on what a DDS is made of. But how it’s made is just as critical, especially if we’re serious about environmental impact. Traditionally, drug delivery systems have relied heavily on:
- Toxic organic solvents (e.g., dichloromethane, chloroform)
- High energy inputs (drying, milling, granulation)
- Multi-step purification with significant waste streams
Green DDS design demands process innovation, ways to create complex systems with less energy, fewer chemicals, and minimal downstream waste.
Supercritical CO₂ Drying
Supercritical carbon dioxide (scCO₂) replaces organic solvents in particle drying. It’s non-toxic, recyclable, and enables gentle drying at low temperatures, ideal for heat-sensitive APIs. It’s already being used in microparticle encapsulation of peptides, vaccines, and probiotics.
Solvent-Free Hot-Melt Extrusion (HME)
HME is a solvent-free technology where polymers and APIs are melted and extruded to form DDS matrices. Not only does it avoid hazardous solvents, it allows:
- Continuous manufacturing
- Immediate solid-state stabilization
- Tailored release profiles
HME is now used in commercial products like Gralise® and Zipsor®, proof that green tech can go mainstream.
Aqueous Nanoprecipitation
Instead of using volatile solvents, some DDS manufacturing processes now use water-based systems for particle formation, especially for nanoparticles and nanosuspensions. This significantly reduces VOC emissions and simplifies cleanup.
The Takeaway: Sustainability Demands Systems Thinking
Green DDS aren’t just eco-friendly. They’re more sophisticated, more targeted, and often more clinically effective. But they require us, formulators, process engineers, QA experts, to think not just in grams and minutes, but in impact over time.
“If your DDS works perfectly in a patient, but damages the water system it’s excreted into, is it really successful?”
We need to move from reactive design (fix it later) to proactive design (solve it now). That’s the real innovation green DDS demands, not just new materials, but a new mindset.
5. Case Studies of Biodegradable DDS in Action
Innovation only matters when it leaves the lab and transforms real lives. In the quest for eco-friendly drug delivery, that transformation is already underway, quietly, globally, and with measurable impact.
Let’s explore three compelling cases where biodegradable DDS are not just theories, but fully functional, life-changing interventions.
5.1 Long-Acting Injectable Contraceptives
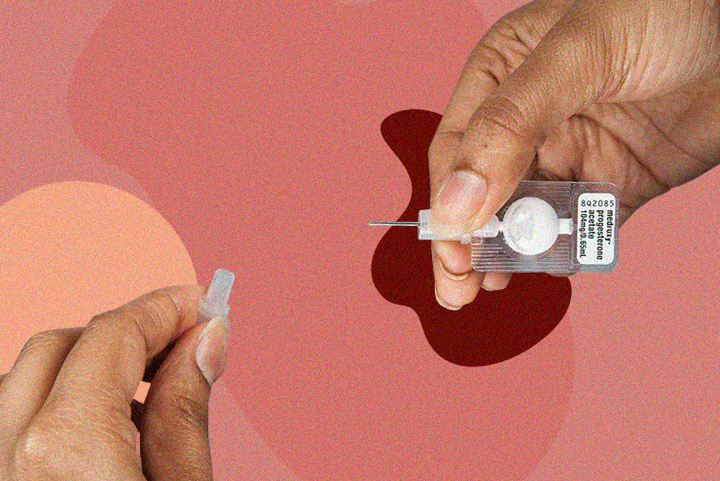
Sayana® Press Contraception Injection
Product: Sayana® Press
- API: Medroxyprogesterone acetate
- Delivery: PLGA-based microsphere suspension in a prefilled auto-disable injector
- Manufacturer: Pfizer Inc.
How It Works
Sayana® Press uses PLGA microspheres as a depot matrix that releases medroxyprogesterone gradually over three months. The biodegradable polymer slowly breaks down into lactic acid and glycolic acid, both safely metabolized by the body. No retrieval. No residual plastic.
Environmental Impact
Unlike traditional multi-dose vials or blistered oral contraceptives, Sayana® Press uses 90% less plastic packaging and requires fewer clinic visits, translating into lower medical waste, reduced transportation emissions, and minimized supply chain footprint.
Why It Matters
In low-resource settings, women often travel long distances to access contraception. Sayana’s once-every-3-month injection reduces burden, increases compliance, and prevents unnecessary environmental waste, especially where incineration or recycling of clinical waste isn’t feasible.
“It’s rare to find a product that respects a woman’s time, a clinician’s efficiency, and the planet’s resources, Sayana Press manages all three.”
5.2 Microneedle Patches for Vaccination
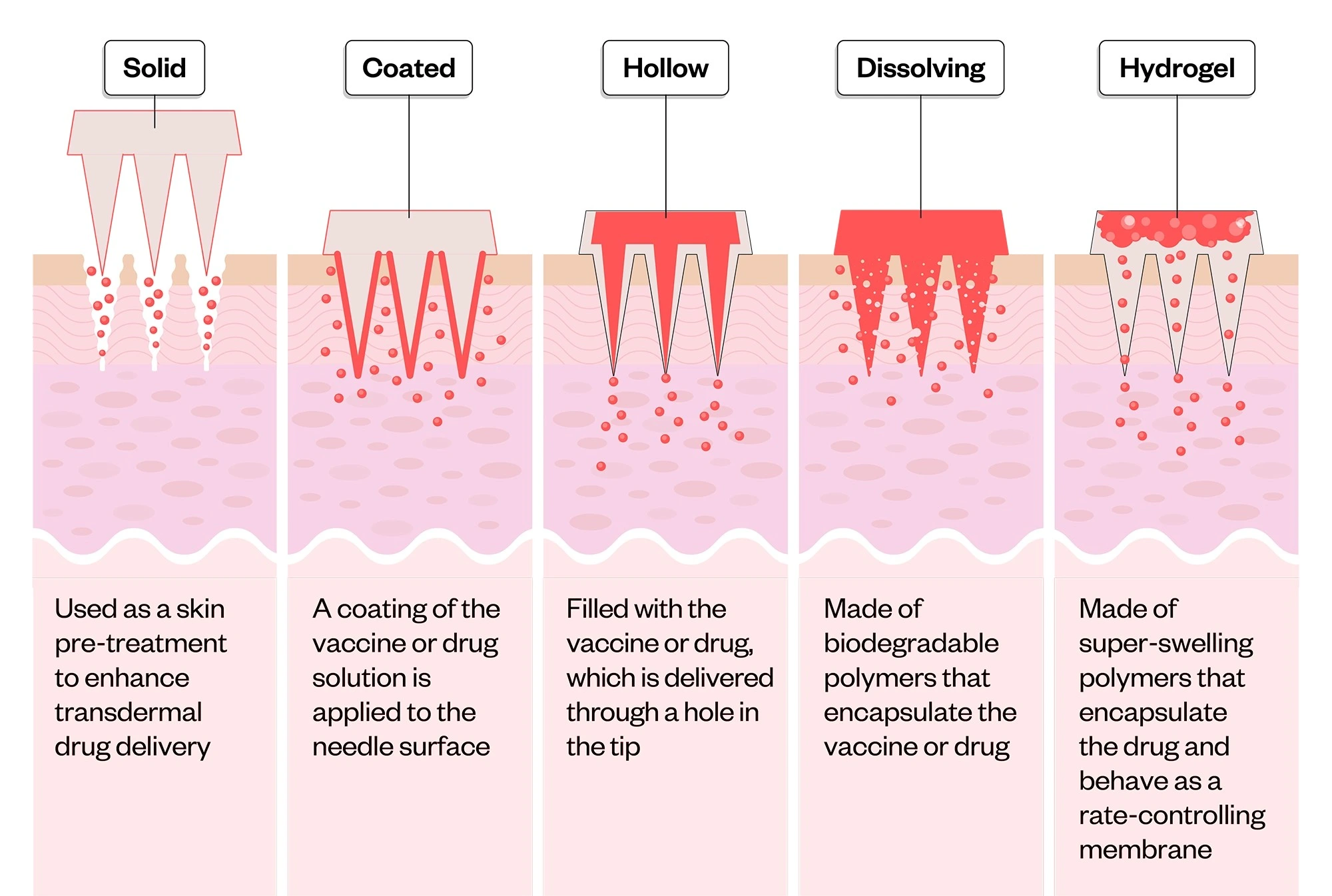
Material: Hyaluronic acid or pullulan-based biodegradable microneedles
- Use Cases: Influenza, COVID-19, measles, HPV vaccines
- Administered: Topically, no syringe, no clinic, no sharps
How It Works
These microneedles are engineered to penetrate just below the stratum corneum, releasing vaccine antigen as they dissolve completely within the skin. The base patch can be discarded as regular waste, no biohazard labeling required.
Eco-Friendly Innovation
Traditional vaccines require:
- Cold-chain storage
- Syringes and needles
- Alcohol swabs
- Sharps containers
Microneedle patches eliminate all of that. They’re thermostable, reducing refrigeration energy use, and completely biodegradable, leaving no trace behind, clinically or ecologically.
Public Health Implications
Microneedles enable mass vaccination campaigns in remote regions, with minimal training. No hazardous waste means easier disposal and dramatically reduced risk of needle-stick injuries, a chronic problem in global immunization programs.
“It’s not just about making vaccines easier, it’s about making them more equitable, safer, and greener.”
5.3 Ocular Inserts for Glaucoma
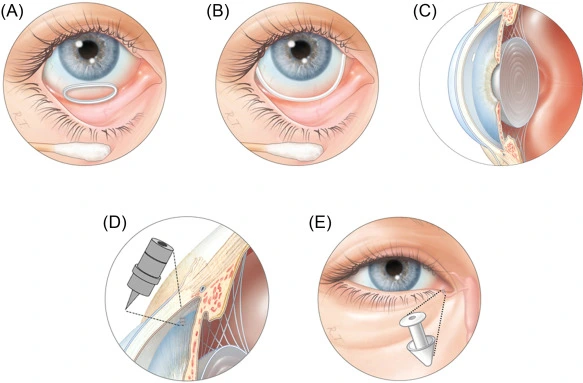
Product Concept: Sustained-release ocular inserts
- Materials: PLGA and hydroxypropyl methylcellulose (HPMC)
- Drug: Timolol, bimatoprost, or latanoprost for intraocular pressure control
How It Works
Patients with glaucoma typically rely on daily eye drops, which suffer from:
- Low bioavailability (often <5%)
- Poor adherence (missed doses, improper administration)
- Frequent plastic bottle waste
Biodegradable ocular inserts are placed in the conjunctival sac, where they gradually dissolve over several weeks, releasing the active compound in a controlled, consistent manner.
Environmental + Clinical Benefits
One patient using daily eye drops can generate over 30 plastic bottles annually, all non-recyclable. Multiply that by millions of glaucoma patients globally, and the waste is staggering. By contrast, biodegradable inserts reduce:
- Plastic medical waste by >90%
- Missed doses and disease progression
- Patient anxiety over complex dosing regimens
“It’s one of those rare innovations that improves both compliance and compassion.”
Real Products. Real Patients. Real Progress.
These aren’t hypotheticals. They are proven examples of how biodegradable DDS can enhance patient outcomes while protecting the planet. And yet, they are just the tip of the iceberg. What they prove is this:
Sustainable formulation is no longer a compromise. It’s an opportunity to create smarter, safer, and more human-centered healthcare systems.
It’s time for us, scientists, regulators, entrepreneurs, to stop treating biodegradability as an afterthought, and start treating it as a core performance metric of every delivery system we design.
6. Challenges in Green DDS Formulation: A Path Paved with Promise, and Friction
For all the enthusiasm surrounding biodegradable drug delivery systems (DDS), the road to widespread adoption is far from smooth. The science is progressing, yes, but translating that progress into viable, commercial products requires navigating a minefield of practical challenges.
Sustainability doesn’t exist in a vacuum. We’re formulating within a complex ecosystem of supply chains, excipient interactions, regulatory standards, and most importantly, patient safety. While the vision of a fully green pharmaceutical future is inspiring, reality demands pragmatism.
Let’s unpack the most pressing challenges holding back biodegradable DDS from becoming the universal standard.
6.1 Material Supply and Scalability: A Green Idea Needs a Green Supply Chain
It’s one thing to identify a biodegradable polymer in the lab, it’s another to source it consistently, at pharmaceutical grade, and at commercial scale.
Natural Polymers: Beautiful but Unpredictable
Natural polymers like chitosan, gelatin, alginate, and plant-derived cellulose are immensely promising. But because they’re derived from biological sources, crustaceans, seaweed, livestock, crops, they suffer from natural variability:
- Moisture content can vary seasonally
- Molecular weight distribution is often inconsistent
- Impurities and bioburden risk differ batch to batch
This variability affects drug loading, release kinetics, and shelf life, forcing formulators to either overdesign or overtest, both time- and cost-intensive strategies.
“No two shrimp shells are the same. But in pharma, reproducibility is sacred.”
Synthetic Biodegradables: Stable but Expensive
Polymers like PLGA, PCL, and PLA offer greater batch uniformity and predictable degradation profiles, a dream from a QA perspective. But they come with a price:
- High production costs
- Specialized polymerization conditions
- Limited suppliers globally
Even as demand grows, economies of scale haven’t yet kicked in, keeping prices out of reach for many generic manufacturers or smaller biotech firms.
This makes one thing clear: Material innovation must be matched with supply chain innovation if green DDS is to become more than a niche capability.
6.2 Drug–Excipient Compatibility: Not Every API Plays Nice
The best DDS in the world is meaningless if it destabilizes the drug it’s supposed to deliver. Biodegradable carriers often introduce new chemical environments, acidic degradation byproducts, moisture-rich matrices, or enzymatic triggers, that can:
- Accelerate API hydrolysis or oxidation
- Denature sensitive biologics
- Alter API polymorphic forms or release profiles
Formulating Biologics is Especially Challenging
Proteins, peptides, and nucleic acids are often incompatible with PLGA-type matrices, because the acid generated during polymer breakdown can lead to:
- Protein aggregation
- Fragmentation
- Activity loss
For small molecules, moisture-sensitive APIs like nitrofurantoin or carbamazepine may suffer degradation when encapsulated in hydrophilic matrices like alginate or gelatin.
“A green excipient that degrades the drug isn’t sustainable, it’s counterproductive.”
Thus, the challenge becomes not just finding a biodegradable material, but ensuring that it degrades on time, but not too soon, and without damaging what it’s supposed to protect. It’s a delicate dance of chemistry and kinetics.
6.3 Regulatory Complexity: Innovation Ahead of Legislation
The biggest elephant in the room? Regulation. Right now, there’s no universal definition, let alone approval pathway for what constitutes a “green” or “eco-friendly” drug delivery system. Different regions are evolving at different speeds, and many regulatory frameworks are still catching up.
No Global Harmonization Yet
- EMA encourages eco-conscious design, but doesn’t offer a standardized evaluation rubric.
- FDA CDER considers environmental impact during NDAs, but it’s typically secondary to safety and efficacy.
- WHO and PIC/S acknowledge the value of biodegradability but stop short of offering compliance guidance.
This creates a regulatory gray zone, where developers:
- May face longer review cycles
- Are required to submit novel data on excipient degradation
- Encounter resistance if they substitute excipients with limited historical use
As one EMA official put it: “Sustainability is encouraged, but patient safety remains non-negotiable.”
This quote reflects a deeper truth: Green DDS is not yet seen as essential, it’s still treated as experimental. Until that mindset changes, the adoption curve will remain slow.
Bridging the Gap: Innovation Demands Infrastructure
What’s needed now is collaborative pressure:
- Academic labs must continue proving concept viability.
- Industry leaders must scale these technologies and share learning.
- Regulators must begin issuing draft guidance on green excipients and lifecycle sustainability assessments.
Until these pieces align, green DDS will remain trapped in a cycle of exception, not expectation. But once they do? We’re looking at nothing short of a paradigm shift, from single-use plastics and passive matrices to intelligent, vanishing systems that serve both the patient and the planet.
7. Future Outlook: Where Green DDS Is Heading
Futuristic lab with AI dashboards, biodegradable implants on a 3D printer, and compostable pill packaging side by side.
If the past decade was about proving biodegradable DDS could work, the next one will be about making them indispensable.
We’re standing at the intersection of formulation science, artificial intelligence, and sustainability, and this convergence is pushing us into uncharted, exciting territory. Green DDS is no longer a fringe innovation. It’s shaping up to be a foundational principle of how we design, manufacture, and deliver therapeutics in a climate-conscious world.
The most visionary developments now taking shape are not only environmentally conscious, they’re intelligent, personalized, and deeply human-centric.
Let’s explore the most promising directions the field is moving in.
7.1 Digital Twins and AI: Making Green Formulation Smarter
For decades, excipient selection was part art, part science, relying heavily on trial-and-error, historical preference, and physical testing. But as the complexity of biodegradable materials increases, that approach becomes too slow, too expensive, and too unpredictable.
Enter AI-powered digital twins, virtual models of drug delivery systems that simulate real-world performance before a single batch is made.
How it works:
- QSAR (Quantitative Structure–Activity Relationships) predict how excipients will behave with certain APIs at the molecular level.
- QSPR (Quantitative Structure–Property Relationships) estimate key physical properties like degradation rate, diffusion, and solubility.
- These models feed into IVIVC simulations, predicting how a DDS will behave from in vitro to in vivo systems, even in different patient populations.
Imagine simulating 10,000 excipient combinations overnight, then choosing the top 3 to validate in the lab. That’s the reality AI offers.
Why it matters for sustainability:
- Fewer failed batches mean less material waste.
- Optimized degradation profiles reduce residual toxicity.
- Reduced animal testing as in silico predictions become more robust.
AI is helping green DDS become not just feasible, but scalable, regulatory-compliant, and globally deployable. It’s not replacing formulation scientists, it’s empowering them to think bigger, move faster, and waste less.
7.2 Fully Compostable Packaging: The Last Mile of Sustainability
Even the most biodegradable drug delivery system becomes problematic when encased in a plastic or foil pack that lasts 400 years. That’s why the frontier of sustainable formulation is expanding beyond the formulation itself, into the packaging.
The innovation:
Startups and R&D divisions across Europe and Asia are developing blister packs made from starch-based bioplastics, bamboo fibers, and mycelium composites. These materials:
- Break down fully in soil or compost within 30–60 days
- Are free from petroleum-based polymers
- Can even carry embedded natural antimicrobial agents to protect contents
Some are designed for OTC supplements, pediatric chewables, and travel-dose medications, where shelf life requirements are shorter and consumer values lean green.
Use Case
One pilot trial in Sweden tested plant-based packaging for children’s probiotics. Not only did it reduce landfill waste by over 80%, but it also doubled consumer trust scores in the brand, based on parental feedback.
In the eyes of today’s consumer, sustainable packaging is no longer a “nice to have.” It’s a moral expectation.
The shift is clear: eco-conscious packaging is not a marketing gimmick, it’s the final step in ensuring a biodegradable product stays truly biodegradable from start to finish.
7.3 Personalized Green Medicine: Where Precision Meets Planet-Conscious Design
The green DDS of the future won’t just be general-purpose carriers, they’ll be tailored to the individual. That means:
- Dosage customized to genetics, weight, and disease state
- Release profiles matched to metabolic rate or microbiome composition
- Biodegradable carriers shaped to fit the patient’s anatomy
And here, 3D printing meets biodegradable polymers in one of the most exciting frontiers in pharmaceutical technology.
Biodegradable 3D Implants
Think antibiotic-eluting bone scaffolds that dissolve after aiding tissue regeneration. Or personalized chemotherapy implants for glioblastoma patients, printed in shapes that match resection cavities post-surgery. By using polymers like PLGA, PCL, or emerging bio-inks based on silk fibroin or alginate blends, these devices:
- Deliver drugs locally (reducing systemic burden)
- Disappear gradually (no second surgery)
- Can be fabricated on-demand for individual patients
The promise here isn’t just ecological, it’s deeply human. Fewer side effects. Greater comfort. And no foreign residue left behind.
Looking Forward: A Sustainable, Personalized, Intelligent Future
We often talk about the future of pharma as digital, personalized, and AI-driven. But the fourth pillar, sustainable, is now inseparable from that vision. Green DDS will soon be:
- Modeled in the cloud before they’re ever made
- Tailored to your genome and environment
- Printed, implanted, and forgotten, as they do their job and gracefully disappear
The convergence of biodegradability, AI, 3D printing, and patient data isn’t a utopian dream. It’s an engineering challenge, one we’re finally prepared to meet.
And perhaps for the first time, we have the tools, the urgency, and the human will to not only treat disease, but to do it in a way that heals our planet, too.
Conclusion: Greener Science, Healthier Future

Where every molecule heals, and none are left to harm. Greener science, for a truly healthier future. Because saving lives and saving the planet should never be at odds.
Biodegradable drug delivery systems are no longer distant ideals or theoretical novelties, they are the very direction in which pharmaceutical science must now move with purpose, urgency, and conviction. This isn’t simply a matter of riding the wave of sustainability; it’s about rethinking what it means to develop medicine in a world where the cost of progress can no longer be the planet itself.
For too long, the industry has focused narrowly on efficacy and compliance, unintentionally leaving behind a trail of plastic waste, persistent polymers, and environmental toxicity. But now, we stand at a turning point, where healing the human body and preserving the health of the planet are no longer two separate goals, but one unified mission.
Biodegradable systems offer more than environmental benefit, they reflect an evolution in our scientific conscience. They embody a shift from disposability to responsibility, from volume to value, and from short-term success to long-term harmony. They challenge us to build smarter, cleaner, more intuitive systems, not just for innovation’s sake, but for future generations who will live with the choices we make today.
And perhaps the most profound truth is this: sustainability in drug delivery doesn’t ask us to compromise efficacy or delay innovation; it asks us to elevate both. It invites us to create solutions that are not only intelligent in function but graceful in disappearance. The kind that heal without harming, treat without leaving a trace, and dissolve not just in the body/ but into the earth, returning as nothing more than water, carbon, and renewal.
If the 20th century was defined by the race to cure disease, then the 21st must be remembered as the era in which we began to cure the damage we left behind in that race. To be a scientist today is to stand not just as a steward of health, but as a guardian of balance. And those who accept this challenge are not just developing the next wave of drug delivery; they are rewriting what it means to deliver medicine at all.
This is our moment. To be precise. To be principled. To be powerful in the quiet, graceful act of doing good, for the patient, for the planet, and for the future. And that, surely, is worth more than applause. It’s worth action.
References
- J. Siepmann & R. Bodmeier (2010). Polymeric Controlled Drug Delivery Systems. International Journal of Pharmaceutics, Volume 384, pp. 165–177.
- [A seminal paper on the science behind polymer degradation and drug diffusion mechanisms.]
- European Medicines Agency (EMA) (2022). Reflection Paper on the Use of Biodegradable Materials in Pharmaceuticals. EMA/CHMP/SWP/731268/2021.
- [Outlines the agency’s perspective on sustainability, patient safety, and excipient qualification.]
- G. R. Maheshwari et al. (2023). Green Drug Delivery Systems: An Emerging Paradigm. Biomaterials Advances, Volume 146, 213297.
- [Recent exploration of eco-friendly systems with emphasis on biodegradable polymers and green excipients.]
- U.S. FDA CDER (2021). Innovative Drug Delivery Systems: Trends and Regulatory Perspective.
- [Highlights challenges and opportunities in approving biodegradable and novel DDS under the FDA framework.]
- K. Jain (2020). Biodegradable Polymers in Controlled Drug Delivery: An Overview. Journal of Materials Science: Materials in Medicine, 31(5): 41.
- [Excellent breakdown of PLGA, PCL, and other synthetic degradable materials.]
- World Health Organization (WHO) (2019). Global Impact of Pharmaceutical Waste on the Environment: Challenges and Policy Recommendations.
- [Framework for environmental sustainability in pharma from a global health lens.]
- DeepChem Documentation (2023).
- DeepChem: Democratizing Drug Discovery with Machine Learning.
- GitHub repository: https://github.com/deepchem/deepchem
- [Open-source tools applied to predictive excipient compatibility and DDS design.]
- D. Wang, Y. Luo, et al. (2021). Stimuli-Responsive Drug Delivery Using Chitosan Derivatives. Carbohydrate Polymers, Volume 261, 117877.
- [Focused on chitosan-based nanoparticles sensitive to pH and enzymatic triggers.]
- Sustainable Packaging Coalition (2022). Designing for Compostability: Bioplastics in Pharma and Health Packaging.
- [Practical guide to implementing biodegradable packaging across the pharmaceutical sector.]
- Langer Lab, MIT – Microneedle Technologies for Transdermal Drug Delivery, Publications 2015–2023.
- [Series of high-impact studies on biodegradable microneedles and translational DDS.]
- C. Li et al. (2022). 3D-Printed Biodegradable Implants for Personalized Drug Delivery. Advanced Functional Materials, Volume 32, 2200039.
- [Explores emerging interfaces between 3D printing and eco-friendly polymers.]
- OECD (2021). Pharmaceutical Residues in Freshwater: Hazard, Risk, and Policy Responses.
- [Comprehensive review of API discharge in aquatic systems and biodegradability challenges.]
- A. Shrestha & B. Shah (2020). Natural Polymers in Biodegradable DDS: Sourcing to Scale-Up. Pharmaceutical Development and Technology, 25(6), 675–686.
- [Critical insight into raw material limitations and standardization challenges.]
- Nature Reviews Drug Discovery (2022). The Green Revolution in Drug Delivery. Vol. 21, pp. 900–902.
- [Industry perspective on integrating environmental metrics in DDS development.]
- United Nations Environment Programme (UNEP) (2020). Emerging Issues of Environmental Concern: Pharmaceutical Pollution.
- [UN guidance shaping regulatory reforms on eco-toxicological considerations.]