Introduction: Why Regulatory Affairs is a High-Impact Career
In the pharmaceutical and biotechnology industries, Regulatory Affairs (RA) professionals serve as the critical link between companies and global health authorities such as the FDA (USA), EMA (Europe), and DRAP (Pakistan). With increasing demand for regulatory compliance, drug safety, and fast-track approvals, Regulatory Affairs roles have become more strategic than ever.
If you’re aiming to break into this rewarding field or advance your career, this interview and career prep guide will help you stand out with confidence.
Regulatory Affairs is a high-impact, fast-growing career in the pharmaceutical and healthcare industries. In 2025, RA professionals are in high demand for their vital role in ensuring drug safety, regulatory compliance, and global product approvals. This career offers strong job stability, excellent salary potential, and diverse specialization areas, including CMC, clinical trials, eCTD submissions, labeling, and regulatory strategy.
With the rise of global regulations (FDA, EMA, WHO), digital health, and AI in drug development, Regulatory Affairs professionals are positioned at the center of innovation and public health protection. The role combines science, policy, and leadership, making it a rewarding, future-proof profession for graduates in pharmacy, life sciences, or biotechnology.

How to Prepare for a Regulatory Affairs Interview in 2025
1. Research the Job Description Thoroughly
Before appearing for any Regulatory Affairs interview, carefully review the job responsibilities and required qualifications.
Example Tip: If the role mentions “ANDA dossier compilation,” be ready to explain the ANDA submission structure and FDA expectations.
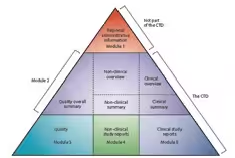
2. Master the CTD Dossier Structure
You’ll likely be asked about Common Technical Document (CTD) modules:
- Module 1 – Regional Administrative Information
- Module 2 – Summaries
- Module 3 – Quality (CMC)
- Module 4 – Non-clinical Study Reports
- Module 5 – Clinical Study Reports
Pro Tip: Use real-life examples of CTD submissions you’ve worked on to demonstrate experience.Your Attractive Heading
3. Practice Common Regulatory Affairs Interview Questions
- Prepare to answer both technical and situational questions:
- What is the difference between NDA and ANDA?
- How would you handle a regulatory query from the FDA?
- What guidelines are followed for stability studies?
- Describe your experience with post-approval changes.
Use the STAR method (Situation, Task, Action, Result) to structure your answers.
Pro Tip: Research recent updates from ICH, WHO, and your regional authority (like DRAP or CDSCO).

4. Revise Key Regulatory Guidelines
- Make sure you’re familiar with:
- ICH Guidelines (Q1-Q14, E2, S1-S9)
- FDA Submission Guidelines
- EMA’s Marketing Authorization Procedures
- WHO Prequalification Process
- Refer to official sites like:
5. Highlight Soft Skills that Set You Apart
In addition to technical expertise, RA roles require:
- Excellent communication (especially for query responses)
- Attention to detail
- Time management
- Cross-functional collaboration
Use specific examples to show how you’ve demonstrated these in academic or industry settings.
6. Research the Company & Recent Regulatory Trends
- Visit the company’s website – check their pipeline, mission, and latest news.
- Understand which markets they operate in (EU, US, GCC, etc.).
- Stay updated with emerging trends in 2025:
- AI in regulatory review
- Electronic submissions (eCTD)
- Regulatory reliance and work-sharing models
General Regulatory Affairs Interview Questions
Basic Conceptual Questions
- These test your foundational knowledge:
- What is Regulatory Affairs? Why is it important in the pharmaceutical industry?
- What is the difference between NDA, ANDA, and IND?
- Explain the Common Technical Document (CTD) structure.
- What are the key responsibilities of a regulatory affairs professional?
- What are the different phases of clinical trials?
- What is an orphan drug, and how is it regulated?
- How is a generic drug approved?
- Why did you choose a career in Regulatory Affairs?
- What do you understand by CTD and eCTD formats?
- What are the main responsibilities of RA during a product’s lifecycle?
- What are the key regulatory agencies globally? Name at least five.
Technical & Documentation-Based Questions
- What are the modules of the CTD dossier?
- What’s the difference between NDA and ANDA?
- How do you compile an eCTD dossier? What software tools have you used?
- What is a CEP, and why is it important?
- Explain the differences between variations (Type IA, IB, Type II) in EU regulations.
Region-Specific Regulatory Questions
- What is the procedure for drug registration in the EU via DCP/MRP?
- Explain the FDA’s 505(b)(1) vs 505(b)(2) application pathways.
- What are the requirements for WHO prequalification of a product?
- Describe the drug approval process in GCC countries.
- How do you handle registration renewals in African or Southeast Asian markets?
Scenario-Based / Problem-Solving Questions
- How would you respond to a deficiency letter from the FDA or EMA?
- What steps would you take if a deadline for a major submission is at risk?
- Describe a situation where your input helped avoid regulatory non-compliance.
- What would you do if there were conflicting data in a clinical or CMC section?
- How do you prioritize when managing multiple dossiers across countries?
Behavioral & Soft Skills Questions
- How do you stay updated with changes in global regulatory guidelines?
- Have you worked in cross-functional teams (e.g., R&D, QA, Marketing)?
- Describe a time when you had to deal with a difficult regulatory authority.
- How do you handle high-pressure situations with tight deadlines?
- Tell us about a mistake you made in a submission and how you corrected it.
For Senior/Managerial RA Positions
- What is your experience with global regulatory strategy development?
- How do you lead a team during a major variation or launch submission?
- What KPIs do you track for regulatory operations efficiency?
- How do you liaise with global consultants or regional affiliates?
- What is your approach to regulatory intelligence and risk management
Career Tips to Grow in Regulatory Affairs
1. Invest in Certifications
- Stand out by pursuing:
- Regulatory Affairs Certification (RAC) by RAPS
- ICH-GCP Training
- eCTD Software Training (Lorenz DocuBridge, Veeva Vault)
These certifications not only enhance your CV but also show recruiters your commitment to excellence.
2. Gain Global Exposure
Even if you work locally, knowledge of international procedures is a career booster. Learn submission strategies for:
- FDA (USA) – 505(b)(1), 505(b)(2), ANDA
- EMA (Europe) – Centralized, Decentralized Procedures
- PMDA (Japan), TGA (Australia)
- WHO PQ for vaccines and generics
3. Build a Strong LinkedIn Profile
- Use your LinkedIn as a dynamic CV. Include:
- CTD projects handled
- Authorities you’ve interacted with
- RA tools/software you know
- Certifications and workshops attended
Bonus Tip: Follow pharma regulatory groups and contribute to discussion.
4. Stay Updated with Regulatory Trends
- Subscribe to:
- Regulatory Focus (RAPS.org)
- FDA Email Alerts
- ICH Newsletter
- PharmExec.com
- NMRA, DRAP, CDSCO portals
- Being informed helps in interviews and keeps your skills relevant.
5. Document Your Achievements
- Keep records of:
- Marketed products
- Countries registered
- Query letters resolved
- Inspection participation
- SOPs drafted or reviewed
- This serves as a portfolio for interviews and promotions.
How to Excel in Your Regulatory Affairs Career
Build a Strong Regulatory Foundation
- Master the basics early on:
- Understand CTD/eCTD dossier formats (Modules 1–5)
- Learn ICH guidelines (Q8, Q9, Q10, E6, etc.)
- Study local and international regulations (DRAP, FDA, EMA, MHRA, WHO)
Gain Hands-on Experience
- Experience matters more than theory. To grow:
- Get involved in dossier preparation and submissions
- Assist in regulatory audits or authority meetings
- Participate in variation, renewal, or labeling updates
Focus on Liaisoning & Networking
- Build relationships with regulators, consultants, and CROs
- Join associations like RAPS, ISoP, or DIA
- Attend industry events and online forums
Liaisoning in Regulatory Affairs
Liaisoning in Regulatory Affairs refers to the process of effective coordination and communication between the pharmaceutical or medical device company and key external and internal stakeholders to ensure regulatory compliance, timely approvals, and successful product lifecycle management.
Importance of Liaisoning
| Benefit | Outcome |
| Timely Approvals | Faster market access |
| Stronger Compliance | Fewer regulatory objections |
| Efficient Workflow | Clear communication between departments |
| Risk Management | Early identification of compliance gaps |
Pursue Higher Education or Certifications
- Enhance your credentials with:
- Postgraduate degrees in Regulatory Affairs, Pharmaceutical Sciences, or Quality Assurance
- Certifications: RAPS RAC, PGDRA, eCTD Submission Specialist, etc.
Stay Updated with Trends in 2025
- AI in regulatory submissions and document tracking
- Regulatory Reliance models in developing countries
- Digital health and medical device regulations
- Changes in DRAP/ICH/EMA/FDA guidelines
Build a Personal Brand
- Share insights on LinkedIn or regulatory forums
- Write short articles or blogs about RA topics
- Create a professional CV and interview pitch
Summary: 20 Quick Tips to Excel in Regulatory Affairs
| Category | Tip |
|---|---|
| Regulatory Knowledge | 1. Stay updated on global regulatory changes (FDA, EMA, PMDA, etc.) |
| 2. Understand ICH guidelines (especially Q, E, M series) | |
| 3. Know regulatory requirements across the product lifecycle | |
| 4. Learn country-specific regulatory frameworks | |
| 5. Familiarize yourself with submission types (IND, NDA, MAA, etc.) | |
| Technology & Tools | 6. Use eCTD and regulatory submission tools effectively |
| 7. Apply AI tools for regulatory intelligence and labeling | |
| 8. Maintain accurate document control and version tracking | |
| 9. Learn to navigate regulatory databases (e.g., FDA’s database) | |
| 10. Keep up with digital transformation in regulatory operations | |
| Communication & Strategy | 11. Improve technical and regulatory writing skills |
| 12. Develop strong responses to Health Authority questions | |
| 13. Coordinate effectively with cross-functional teams | |
| 14. Present regulatory risks clearly to decision-makers | |
| 15. Tailor communication style for regulators vs internal teams | |
| Career Development | 16. Earn recognized certifications (e.g., RAC, RAPS training) |
| 17. Attend industry conferences and webinars regularly | |
| 18. Join regulatory affairs associations and peer groups | |
| 19. Study real-world case studies and compliance failures | |
| 20. Engage in continuous professional development and learning |
This guide is especially useful for:
Aspiring regulatory professionals preparing for interviews or certifications
Mid-level specialists seeking to sharpen skills or move into leadership roles
Cross-functional team members transitioning into Regulatory Affairs (e.g., from QA, Clinical)
Global RA professionals navigating multi-region compliance challenges
Conclusion
A career in Regulatory Affairs is both meaningful and impactful, offering opportunities in pharma companies, biotech firms, consultancies, and regulatory agencies worldwide.
To succeed, focus on building both technical knowledge and soft skills, stay updated with global guidelines, and prepare thoroughly for every interview. Whether you’re starting or looking to level up, these interview preparation tips and career strategies will position you as a top candidate in the competitive pharmaceutical sector.
Final Word: Position Yourself as a Top Candidate
In 2025 and beyond, Regulatory Affairs professionals who combine up-to-date technical knowledge, effective communication skills, and a proactive mindset will be highly sought after. By preparing strategically for interviews and continuously investing in your learning, you can build a thriving, globally relevant career that directly contributes to healthcare innovation and safety.
References (for further reading)
- ICH Guidelines: www.ich.org
- FDA Guidance Portal: www.fda.gov
- EMA Regulatory Updates: www.ema.europa.eu
- WHO Prequalification: www.who.int