Continuing our detailed preformulation study series, this article (Part II) builds on the foundation laid in Part I, where we explored the core physicochemical properties affecting drug formulation. Part II focuses on critical aspects such as solubility analysis, pKa and ionization constants, partition coefficient, dissolution behavior, and comprehensive stability assessments.
These elements play a pivotal role in predicting bioavailability, ensuring formulation robustness, and complying with regulatory standards. A thorough preformulation study at this stage enables formulation scientists to anticipate challenges and optimize drug delivery systems effectively. Whether you’re navigating early R&D or refining an existing product, mastering these principles is essential to successful pharmaceutical development.
Solubility Analysis in Preformulation Study
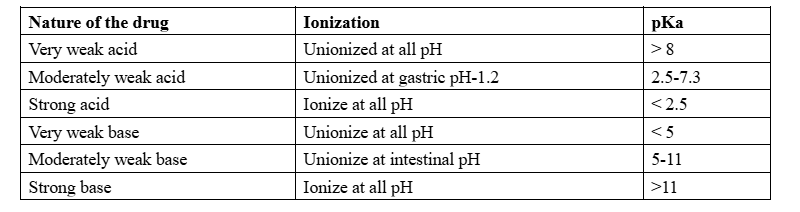
pKa ionization constant
Ionization constant: An ionization constant (K) is an equilibrium constant that measures the degree to which a substance ionizes or breaks apart into ions in a solution. It indicates the ratio of the concentrations of the ions to the concentration of the undissociated substance when equilibrium is reached.
- Weak base: a base that gives a 10% or less yield of hydroxide ions when dissolved in water.
- Weak acid: an acid that gives a 10% or less yield of hydronium ions when dissolved in water.
Dissociation constant (K ): A constant that depends upon the equilibrium between the ions and the molecules that are not ionized in a solution or liquid
The degree of a drug’s ionization depends both on the pH of the solution in which it is presented to the biologic membrane and on the pKa, or dissociation constant, of the drug (whether an acid or base). The concept of pKa is derived from the Henderson–Hasselbalch equation.
Determining the pKa of a drug based on its nature:
For acidic compounds:
PH = pKa + log ([ionized drug]/[un-ionized drug])
For basic compounds:
PH = pKa + log ([un-ionized drug]/[ionized drug]
Solubilization
The highest quantity of solute that can dissolve in one unit of solution to create a saturated solution at a specific temperature is referred to as the solubility of the drug. Solubilization is the method of enhancing the apparent solubility of a poorly soluble substance by embedding it into micelles, thus creating a thermodynamically stable solution. The capacity of a surfactant solution to dissolve or solubilize compounds that are water-insoluble or have limited solubility begins at the.
According to the Biopharmaceutical Classification System (BCS), based on solubility and permeability, drugs are classified into the following four groups:
The classification mentioned above states that a dosage form is considered extremely soluble if the maximum dose dissolves in fewer than 250 milliliters of water with a pH between 1 and 7.5.
Furthermore, if a human absorbs more than 90% of a dose, the dosage form is said to be very permeable. However, if more than 85% of the indicated amount of the medication component dissolves in 30 minutes, the dose form is said to be quickly dissolving. A compound is not regarded as having dissolution-related absorption issues if its aqueous solubility is more than 1% w/v.
Rules of thumb
- In polar liquids, polar solutes dissolve.
- In non-polar liquids, non-polar solutes dissolve.
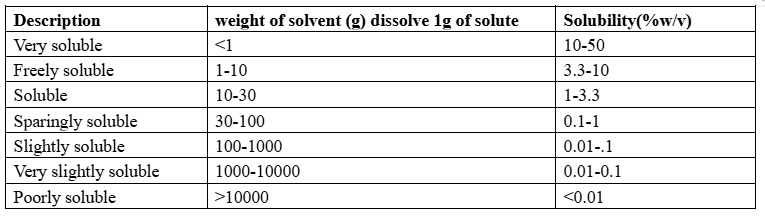
Determination of solubility
Partition Coefficient
The lipophilicity of an organic molecule is typically characterized by a partition coefficient, log P, defined as the ratio of the concentration of the neutral compound at equilibrium in both organic and aqueous phases. One way to evaluate a drug’s lipophilicity and its capability to penetrate cell membranes is through the oil/water partition coefficient in systems like octanol/water and chloroform/water.
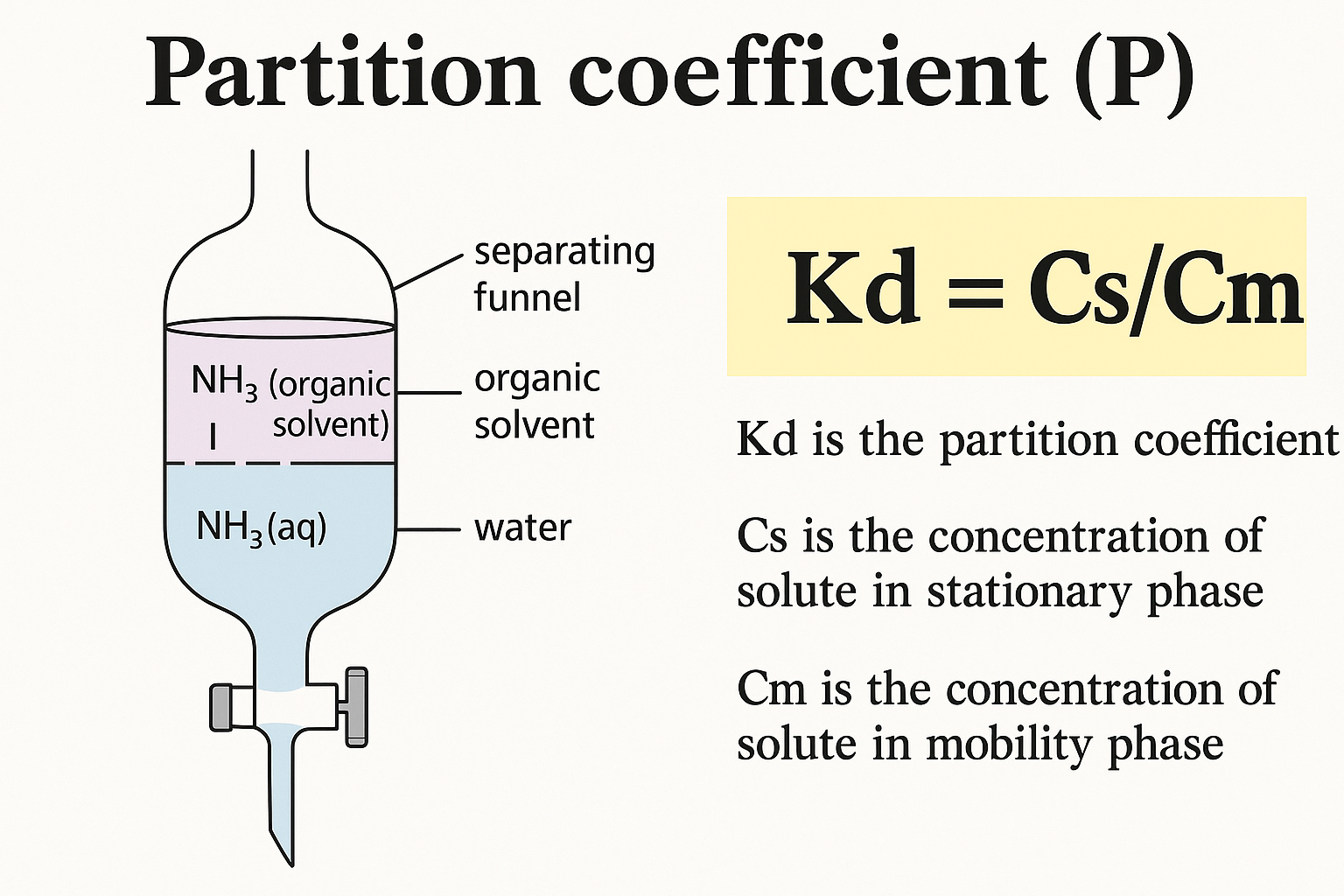
Mathematically, it is represented as
Po/w = (Conc. of the drug in organic phase/ Conc. of the drug in water) equilibrium.
- Compounds with log P values between 1 and 3 show good absorption
- Log P values greater than 6 or less than 3 often have poor transport characteristics.
- Highly non-polar molecules have a preference to reside in the lipophilic regions of membranes, and very polar compounds show poor bioavailability because of their inability to penetrate membrane barriers
Ksp (common ion effect)
The common-ion effect describes the reduction in solubility of an ionic compound when a soluble substance that shares an ion with the precipitate is introduced into the solution.
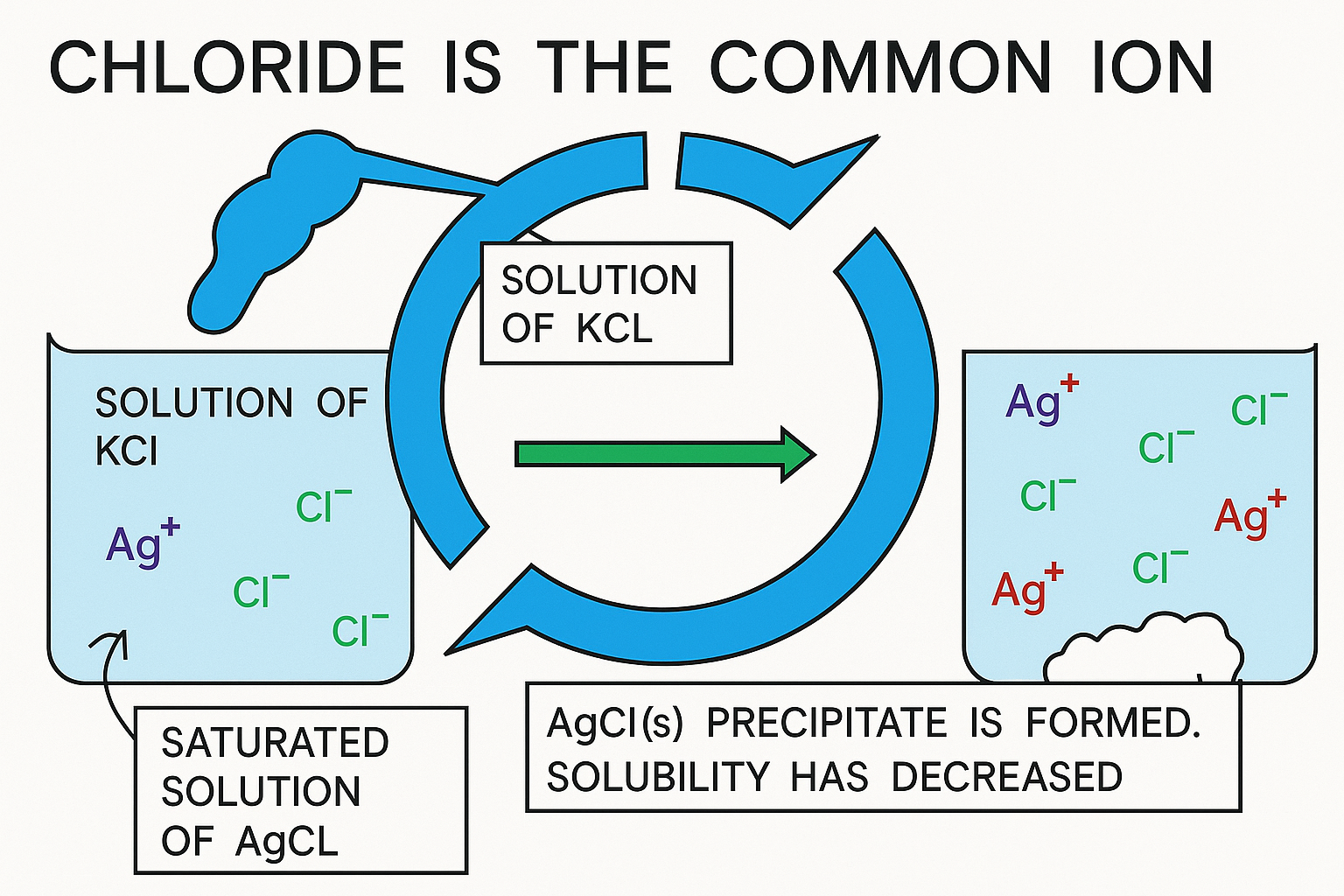
This phenomenon is explained by Le Chatelier’s principle regarding the equilibrium of ionic association and dissociation reactions. It is most commonly observed as a decrease in solubility for salts and other weak electrolytes. When the concentration of one of the ions from the salt is increased, it causes more precipitation, which results in a lower concentration of both ions until the equilibrium of the salt’s solubility is achieved. This outcome occurs because both the original salt and the added compound share a common ion.
Effect of Temperature on Solubility
The solubility of a solute in a solvent is influenced by several factors, including temperature, solute type, and solvent type. When a mole of solute dissolves in a large volume of solvent, the process involves an energy change known as the heat of solution, which may be either absorbed or released.
For most compounds, the dissolution process is endothermic, meaning they absorb heat. As a result, an increase in temperature generally leads to greater solubility for these substances. Conversely, some compounds undergo exothermic dissolution, releasing heat into the surroundings. For such compounds, increasing the temperature reduces their solubility.
It is important to manage temperature carefully during drug formulation, as excessive heat can degrade the active ingredients or alter the properties of the solution. For example, overheating a sucrose solution can lead to the formation of inverted sugar, which can affect the product’s stability and performance.
Dissolution
The dissolution rate refers to the amount of drug substance that dissolves per unit of time under standardized conditions—specifically, at a controlled temperature, with defined solvent composition, and at the liquid/solid interface. This parameter is crucial in assessing how efficiently a drug is released from its dosage form.
In vitro dissolution testing provides valuable insights into a drug product’s potential in vivo performance, especially for orally administered formulations. For drugs classified under the Biopharmaceutical Classification System (BCS) Class I (high solubility, high permeability) and Class III (high solubility, low permeability), dissolution testing can often serve as a predictor of bioavailability.
Preformulation scientists rely on dissolution studies to evaluate the effects of particle size, surface area, and excipients on drug release. This analysis also helps identify whether dissolution is the rate-limiting step in the drug’s absorption process, thereby informing strategies to enhance therapeutic effectiveness and formulation quality.
Stability Analysis in Preformulation Study
Toxicology formulations’ stability
The analysis of stability in toxicology formulations is a vital component of preclinical drug development. It guarantees that the formulations utilized in toxicology studies retain their integrity, potency, and uniformity for the entire duration of the study. This is crucial for producing trustworthy and significant toxicity data, which has a direct effect on the safety evaluation of a drug candidate. This ensures that the formulation stays safe, effective, and consistent throughout its shelf life.
Why is it important?
- Accurate Dosing
- Reliable Toxicity Data
- GLP Compliance
- Formulation Integrity
Stability of the solution
The stability of a solution is an essential element of stability analysis, particularly for liquid pharmaceutical formulations and analytical solutions utilized in testing. It refers to how well a solution can retain its original chemical and physical properties within defined limits during its designated storage and usage duration. These assessments should consider factors such as pH, ionic strength, co-solvents, exposure to light, temperature, and oxygen levels. Compared to the solid state of the drug, degradation in solution form occurs at a notably higher rate.
Why is Solution Stability Important?
- Accurate Results
- Dosage Accuracy
- Prevention of Degradation Products
- Physical Integrity
Decomposition in the solution phase happens via hydrolysis, which follows second-order kinetics, with the order of hydrolysis being Lactam > Ester > Amide > Imide. The primary cause of drug instability is likely attributable to reactions occurring. Water is a key participant, often passively acting as a solvent facilitating interactions between two reactive species, as seen in examples such as anesthetics, antibiotics, vitamins, and barbiturates.
pH-Rate Profile and Its Role in Solubility and Stability
The equilibrium solubility of a drug, commonly referred to as solubility, is defined as the concentration of the drug in solution at a specific point when the solution is saturated. For drugs containing ionizable groups, the solubility of the non-ionized form is referred to as the intrinsic solubility (So).
This distinction is important because ionizable drugs dissociate to varying degrees depending on the pH of the surrounding medium, significantly impacting their overall solubility. If the chemical structure of the compound is known, one can typically predict whether solubility is pH-dependent. However, if the structure is unknown, solubility studies conducted across a range of pH values can help identify the presence of ionizable functional groups.
During preformulation, drug samples in solution are often subjected to forced degradation under extreme pH conditions—typically using 0.1 N HCl or 0.1 N NaOH at 90°C—to understand their stability profiles. These studies help predict degradation pathways, support formulation development, and ensure the selection of appropriate storage and handling conditions.
Importance of Stability Analysis
- Determining Degradation Pathways
- Formulation Development
- Predicting Stability
- Stability-Indicating Method Development
- Regulatory Compliance
- Optimization of Manufacturing Processes
- Understanding In Vivo Behavior
Solid-state stability
Solid-state stability analysis involves assessing the physical and chemical consistency of a drug substance or drug product in its solid state over time when subjected to different environmental conditions. This is a vital component of pharmaceutical development, as the majority of drug products are created and stored in solid forms (such as powders, tablets, and capsules).
Why is Solid-State Stability Important?
- Maintaining Drug Quality
- Preventing Degradation
- Ensuring Efficacy and Safety
- Optimizing Formulation
- Determining Shelf Life
- Regulatory Compliance
Analytical Techniques Used in Stability Analysis
Various analytical techniques are employed to monitor the solid-state stability of drugs, including:
- High-Performance Liquid Chromatography (HPLC)
- Gas Chromatography (GC)
- Mass Spectrometry (MS)
- Spectroscopy (UV-Vis, IR, NMR, Raman)
- X-ray Powder Diffraction (XRPD)
- Thermal Analysis (DSC, TGA)
- Karl Fischer Titration
- Particle Size Analysis
- Dissolution Testing
- Visual Inspection
Conclusion
Preformulation studies represent a crucial phase in the development of pharmaceuticals, offering vital physicochemical, biopharmaceutical, and stability information about the drug substance. This data is key for logical formulation design, facilitating the choice of suitable excipients, enhancing manufacturing processes, forecasting in vivo behavior, and ultimately ensuring the creation of a safe, effective, and stable drug product with a specified shelf life. By comprehensively characterizing the drug substance at an early stage of development, Preformulation studies play a significant role in shortening development timelines, reducing the likelihood of formulation challenges, and improving the overall success rate of pharmaceutical product development.
References
-Komal Bhusare, et al. (2024). Preformulation Studies An Overview. INTERNATIONAL JOURNAL OF PHARMACEUTICAL SCIENCES.
-MANGESH G. BHISE, et al. (2014). A REVIEW ON PREFORMULATION STUDIES. Journal of Emerging Technologies and Innovative Research.
-Priyanka Maurya, et al. (2016). Pharmaceutical polymorphism: The phenomenon affecting the performance of a drug. World Journal of Pharmaceutical Sciences.


