Pharmacoeconomics, a vital health economics discipline, evaluates the economic value of pharmaceutical products and services. This article focuses on Cost-Benefit Analysis (CBA), exploring its principles, methodologies, applications, and challenges in drug evaluation. CBA quantifies drug intervention costs and benefits in monetary terms, enabling direct comparisons across diverse healthcare programs and facilitating optimal resource allocation.
The article also discusses various types of costs and benefits, the importance of different societal perspectives, and ethical considerations in monetizing health outcomes. We examine CBA’s interplay with other pharmacoeconomic analyses, including Cost-Effectiveness Analysis (CEA), Cost-Utility Analysis (CUA), and Cost-Minimization Analysis (CMA). We highlight the critical role of pharmacoeconomics in informing healthcare policy, drug pricing, and reimbursement decisions, as well as promoting the efficient and equitable use of limited healthcare resources. This review provides a thorough understanding of CBA within pharmacoeconomics, underscoring its significance in navigating the complex economic landscape of modern healthcare.
Introduction: The Imperative of Pharmacoeconomics in Modern Healthcare
Modern healthcare systems face escalating costs and finite resources. The pharmaceutical sector, a major healthcare expenditure contributor, demands rigorous evaluation to ensure drug value justifies cost. Pharmacoeconomics, a specialized health economics field, systematically analyzes the economic impact of pharmaceutical products and services. Its objective is to inform decision-making by comparing costs and consequences of drug interventions. By applying economic principles, pharmacoeconomics optimizes resource allocation, enhances patient outcomes, and promotes healthcare system sustainability. It balances financial outlays with health benefits, making it indispensable for policymakers, providers, and pharmaceutical companies.
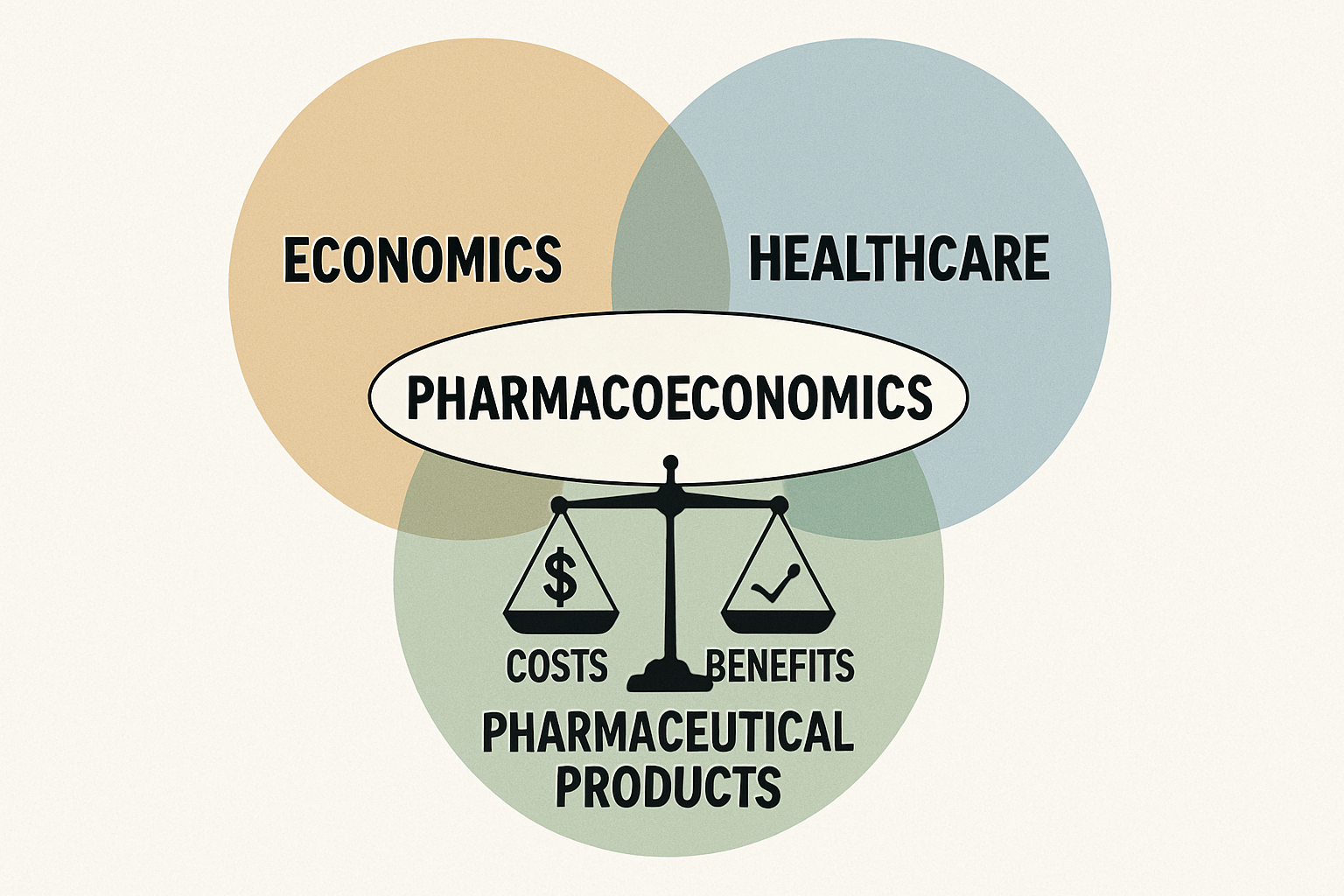
Understanding Cost-Benefit Analysis (CBA) in Pharmacoeconomics
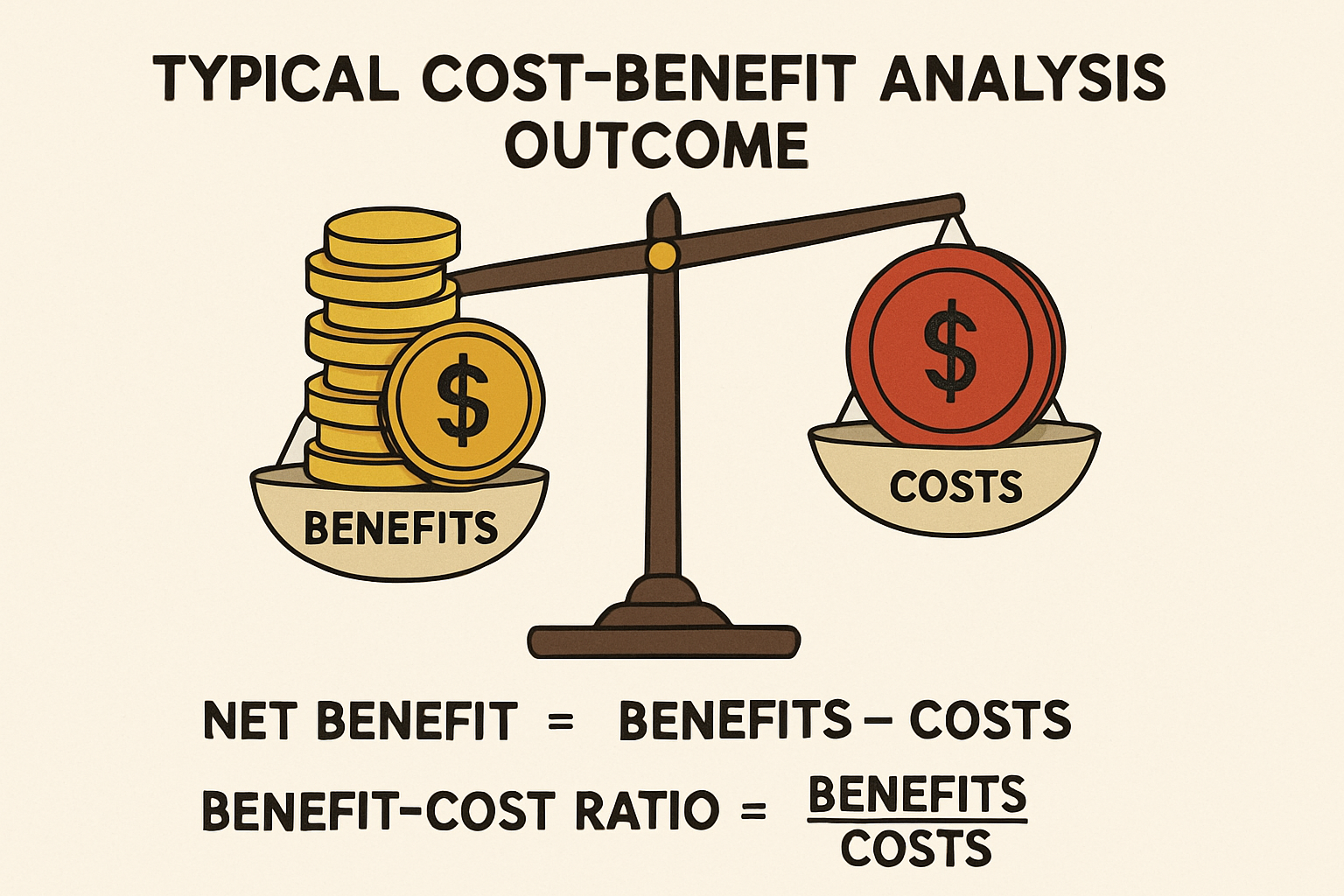
Cost-Benefit Analysis (CBA) is a comprehensive pharmacoeconomic method that quantifies both costs and benefits of healthcare interventions, like drug therapy, in monetary terms. Unlike other analyses, CBA expresses all consequences in a common monetary metric, allowing direct comparison of diverse interventions across health areas. Its strength lies in determining if monetary benefits outweigh costs. If benefits exceed costs, the intervention is economically desirable. This clear decision rule makes CBA a powerful tool for maximizing societal welfare from limited healthcare budgets. CBA provides a robust framework for evaluating the economic viability and societal impact of new and existing drug therapies.
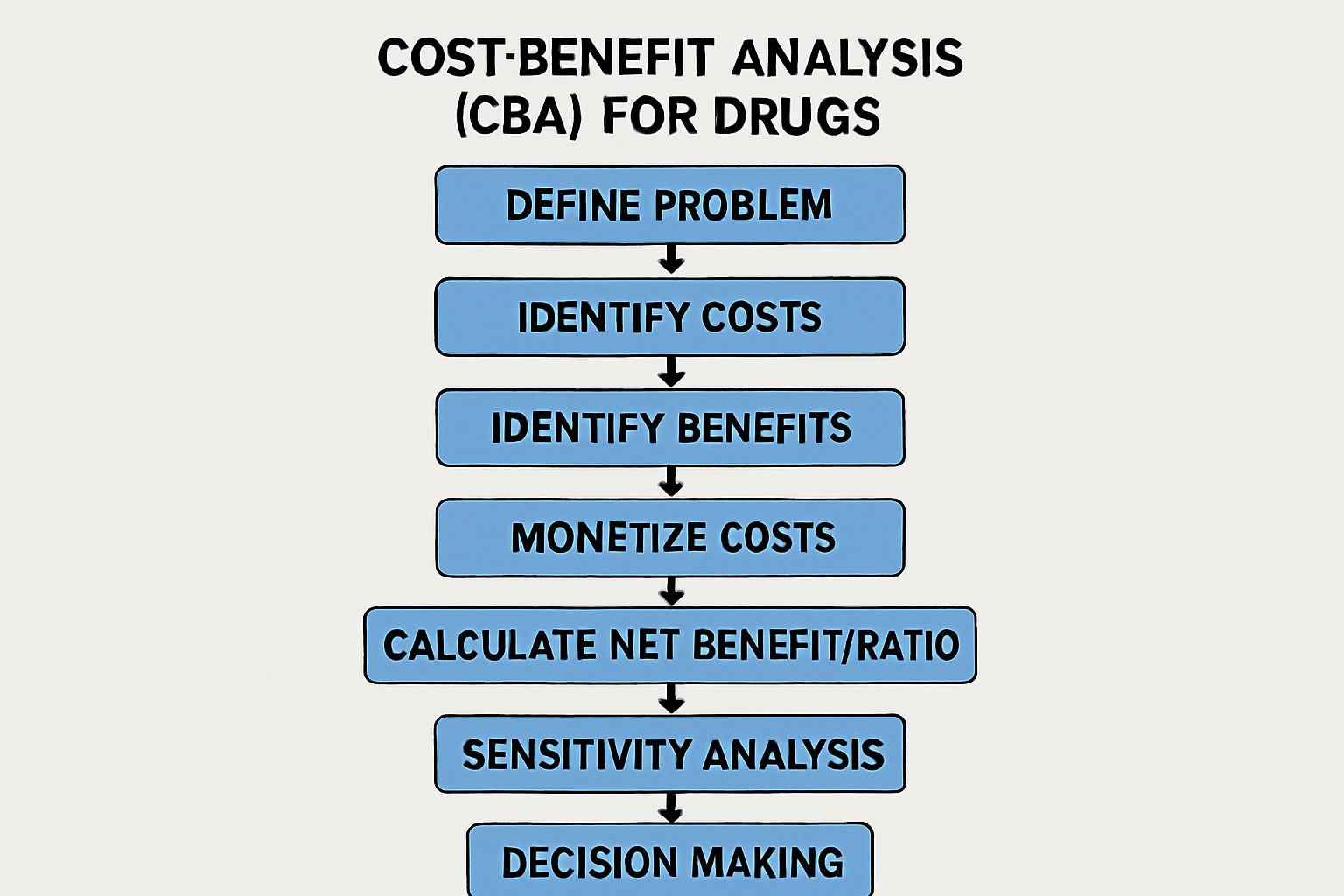
Methodological Steps in Conducting a CBA for Drugs
Conducting a robust CBA in pharmacoeconomics involves systematic steps:
- Define Problem and Objectives: Clearly articulate the drug intervention, comparator, and decision-making context. Identify the analysis perspective.
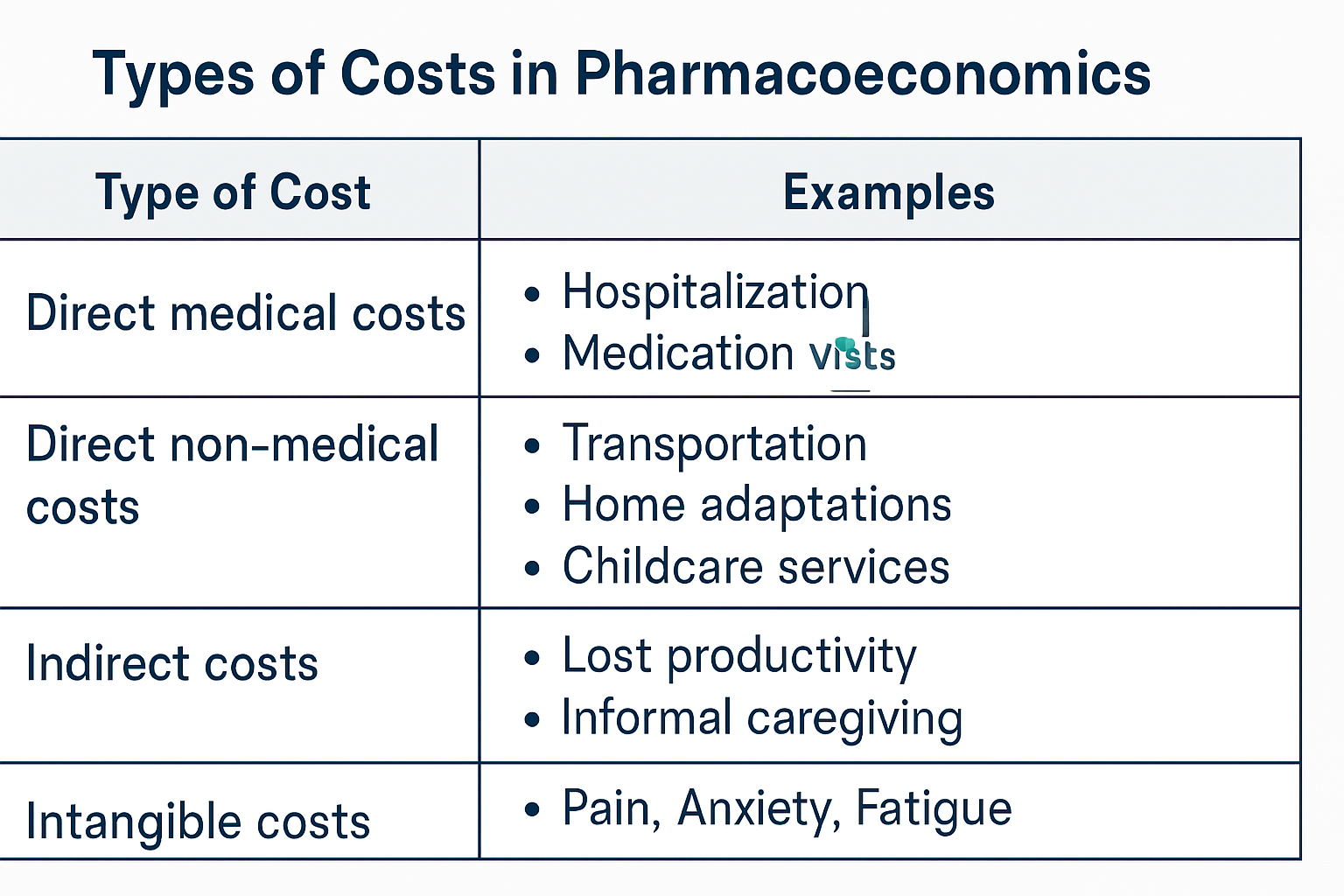
- Identify and Measure Costs: Quantify all relevant costs: direct medical, direct non-medical, indirect, and sometimes intangible costs.
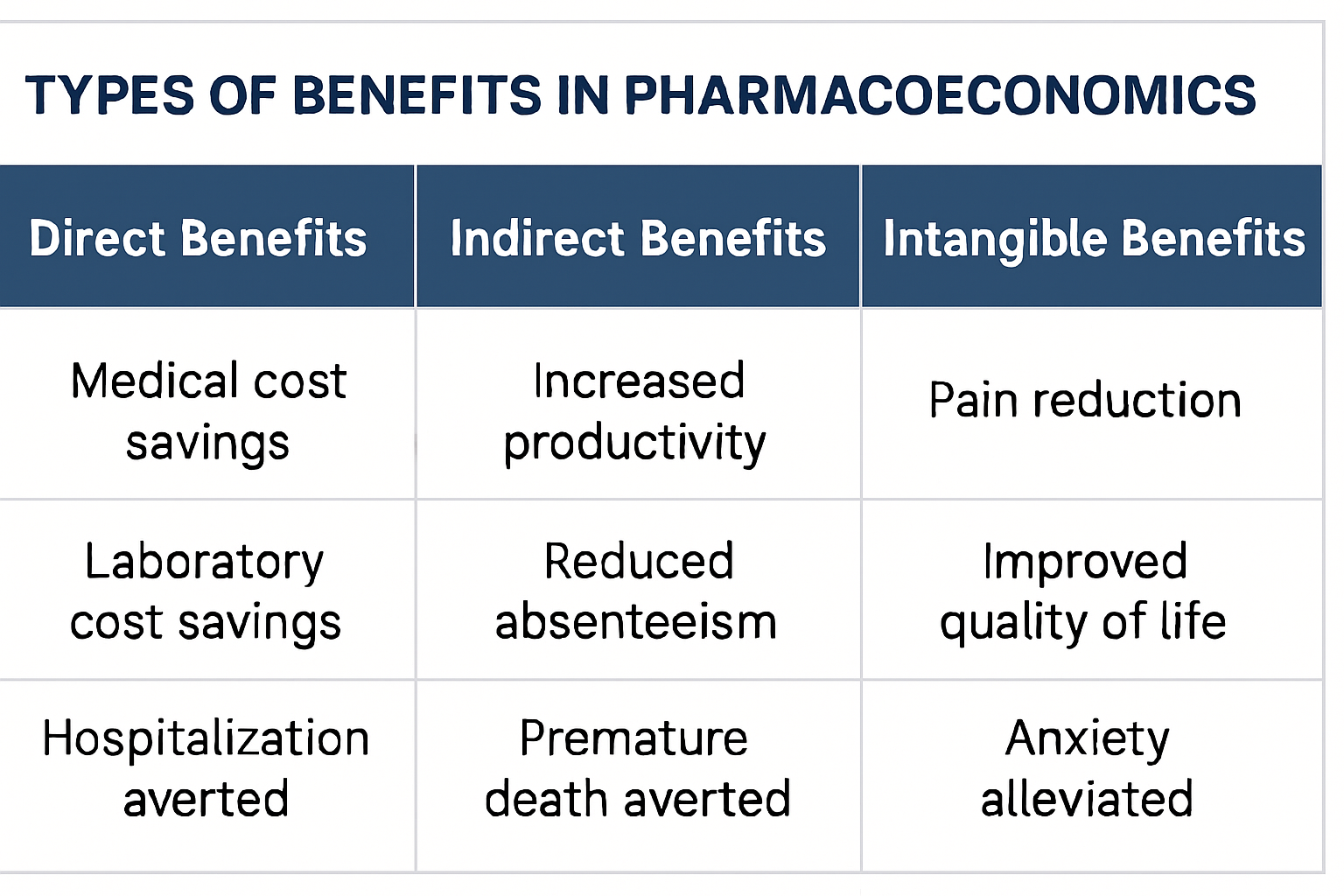
- Identify and Measure Benefits: Identify all relevant benefits: direct, indirect, and intangible benefits. Assign monetary value to these benefits.
- Monetize Costs and Benefits: Monetize costs using market prices. Monetize health benefits using techniques like the human capital approach, willingness-to-pay (WTP), and contingent valuation.
- Calculate Net Benefit or Benefit-Cost Ratio: Calculate net benefit or benefit-cost ratio. A positive net benefit or ratio greater than one indicates benefits outweigh costs.
- Conduct Sensitivity Analysis: Vary key assumptions and parameters to assess impact on results, evaluating robustness of findings.
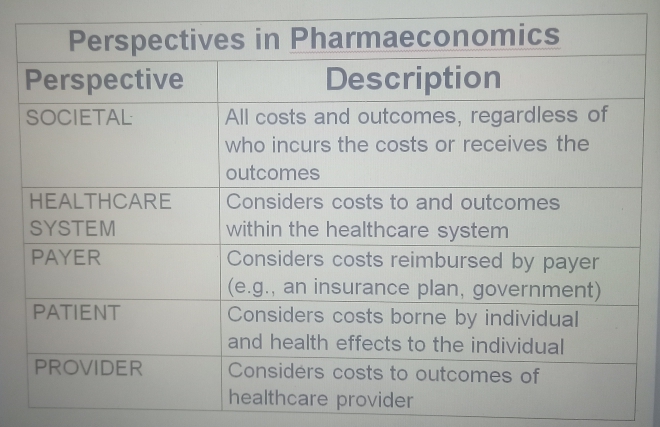
Perspectives in Pharmacoeconomics: Whose Costs and Benefits Count?
The perspective of a pharmacoeconomic analysis significantly influences cost and benefit identification and measurement.
Different stakeholders bear different costs and accrue different benefits. Common perspectives include:
- Societal Perspective: Broadest perspective, considering all costs and benefits relevant to society, regardless of who incurs them. Often preferred for policy decisions.
- Healthcare System Perspective: Focuses on costs and benefits within the healthcare system. Primarily includes direct medical costs.
- Payer Perspective: Considers costs reimbursed by the payer (insurance, government). Includes drug costs, hospitalization, and covered physician visits.
- Patient Perspective: Focuses on costs borne by the patient and individual health effects.
- Provider Perspective: Considers costs and outcomes relevant to the healthcare provider.
The chosen perspective must be explicitly stated, as it dictates included costs and benefits. For comprehensive drug CBA, the societal perspective is often preferred for its full economic impact.
Types of Pharmacoeconomic Analyses: A Comparative Overview
While this article focuses on CBA, it’s crucial to understand its relation to other pharmacoeconomic methods:
- Cost-Minimization Analysis (CMA): Used when interventions have equivalent outcomes. CMA identifies the least costly alternative. CMA is simple but requires strong evidence of equivalent efficacy.
- Cost-Effectiveness Analysis (CEA): Compares intervention costs with outcomes measured in natural health units. Results are expressed as a cost-effectiveness ratio. CEA is widely used when health outcomes are clinically meaningful but not easily monetized.
- Cost-Utility Analysis (CUA): A special CEA type where outcomes are measured in Quality-Adjusted Life Years (QALYs). QALYs combine quantity and quality of life into a single metric. CUA is useful for comparing interventions affecting both life length and quality.
Challenges and Ethical Considerations in Cost-Benefit Analysis of Drugs
Challenges and Ethical Considerations in Cost-Benefit Analysis of Drugs:
- Monetization of Health Outcomes: Assigning monetary values to health benefits is challenging. Techniques like Willingness-to-Pay (WTP) can be controversial and may not reflect societal values.
- Ethical Concerns: Monetizing human life or health raises profound ethical questions. Critics argue health is an intrinsic good not subject to economic valuation.
- Discounting: Discounting future costs and benefits to present value. The choice of discount rate significantly impacts results.
- Uncertainty and Sensitivity: CBAs rely on assumptions and estimates, leading to uncertainty. Robust sensitivity analysis is crucial.
- Distributional Concerns: CBA maximizes overall societal welfare but may not address equity in cost/benefit distribution.
- Data Availability and Quality: Reliable data on all relevant costs and benefits can be hard to obtain.
Addressing these requires transparent methodologies, ethical consideration, and acknowledging model limitations. Pharmacoeconomics aims to refine methods for robust and ethically sound drug evaluations.
The Role of Pharmacoeconomics in Healthcare Policy and Decision-Making
Pharmacoeconomics plays a crucial role in shaping healthcare policy and guiding decision-making:
- Drug Pricing and Reimbursement: Provides evidence for negotiations between pharmaceutical companies and payers on drug pricing and reimbursement.
- Formulary Management: Informs decisions on which drugs to include in formularies, prioritizing cost-effective therapies.
- Resource Allocation: Assists policymakers in efficiently allocating limited healthcare budgets.
- Clinical Guidelines Development: Incorporates pharmacoeconomic evidence into guidelines, supporting evidence-based decisions.
- Health Technology Assessment (HTA): A core component of HTA, evaluating medical, social, ethical, and economic implications of health technologies.
Integrating pharmacoeconomic principles is essential for sustainable, equitable, and high-quality healthcare systems, ensuring drug investments yield maximum health benefit
Future Trends in Pharmacoeconomics and Cost-Benefit Analysis
Pharmacoeconomics continually evolves to address the complexities of modern healthcare. Key future trends include:
- Personalized Medicine and Pharmacogenomics: Adapting analyses to evaluate personalized treatments based on genetic profiles.
- Real-World Evidence (RWE): Increasing use of RWE from electronic health records and registries to complement clinical trial data.
- Value-Based Pricing and Outcomes-Based Agreements: Moving towards models where drug prices link to clinical outcomes.
- Artificial Intelligence and Big Data: AI and big data analytics enhance pharmacoeconomic research efficiency and accuracy.
- Global Health Economics: Growing role in global health, ensuring equitable access to essential medicines.
- Patient-Reported Outcomes (PROs): Greater emphasis on incorporating PROs to capture patient perspectives on drug benefits.
These trends indicate a future where pharmacoeconomics, especially CBA, will be even more integral to healthcare decision-making, driving efficiency, equity, and innovation in drug therapy delivery.
Conclusion: The Enduring Value of Pharmacoeconomics
Pharmacoeconomics, with Cost-Benefit Analysis at its core, is an indispensable discipline in healthcare. It provides tools to evaluate the economic value of drug therapies, ensuring efficient and equitable resource allocation. By monetizing costs and benefits, CBA offers a unique advantage in comparing programs and informing policy. Despite challenges in monetizing health outcomes, continuous evolution and new technologies are leading to more robust evaluations. As healthcare systems strive for sustainability and improved patient outcomes, pharmacoeconomic insights will remain paramount, guiding drug development, pricing, reimbursement, and utilization to maximize societal welfare. Its enduring value lies in its commitment to evidence-based decision-making, ensuring drug investments contribute meaningfully to population health.
Key Takeaways
- Pharmacoeconomics evaluates drug value through cost-benefit analysis.
- Cost-benefit analysis compares drug costs to health outcomes.
- Pharmacoeconomics supports decision-making for drug approval and reimbursement
References
- Drummond, M. F., Sculpher, M. J., Claxton, K., Stoddart, G. L., & Torrance, G. W. (2017). Methods for the economic evaluation of health care programmes (4th ed.). Oxford University Press.
- Neumann, P. J., Cohen, J. T., & Weinstein, M. C. (2018). Updating cost-effectiveness—the curious resilience of the $50,000-per-QALY threshold. New England Journal of Medicine, 378(4), 366–369. https://doi.org/10.1056/NEJMp1711717
- Garattini, S., & Bertele, V. (2019). Cost-benefit analysis in pharmacoeconomics: Principles and applications. Pharmacoeconomics & Outcomes News, 124(3), 10–12.
- Sanders, J. E., & Neumann, P. J. (2020). Pharmacoeconomic evaluations: A systematic review. Value in Health, 23(5), 559–565. https://doi.org/10.1016/j.jval.2020.02.007
- World Health Organization. (2021). Guidelines on health economic evaluation of medicines. WHO Press.
- Smith, A., & Lee, K. (2023). Advances in cost-benefit analysis methodologies for drug evaluation. Journal of Pharmacoeconomics and Outcomes Research, 13(2), 89–102. https://doi.org/10.1016/j.jpor.2022.12.005