What is the pharmaceutical excipient?
A Pharmaceutical excipient is an inactive element, distinct from the active pharmaceutical ingredient(s), used in manufacturing a pharmaceutical product to improve its function. An excipient’s main goal is to ensure the required biological and physicochemical qualities of the medicinal product are satisfied. Excipients in formulations are sometimes known as supplementary ingredients. A clear knowledge of excipients will help a formulator during the preformulation study.
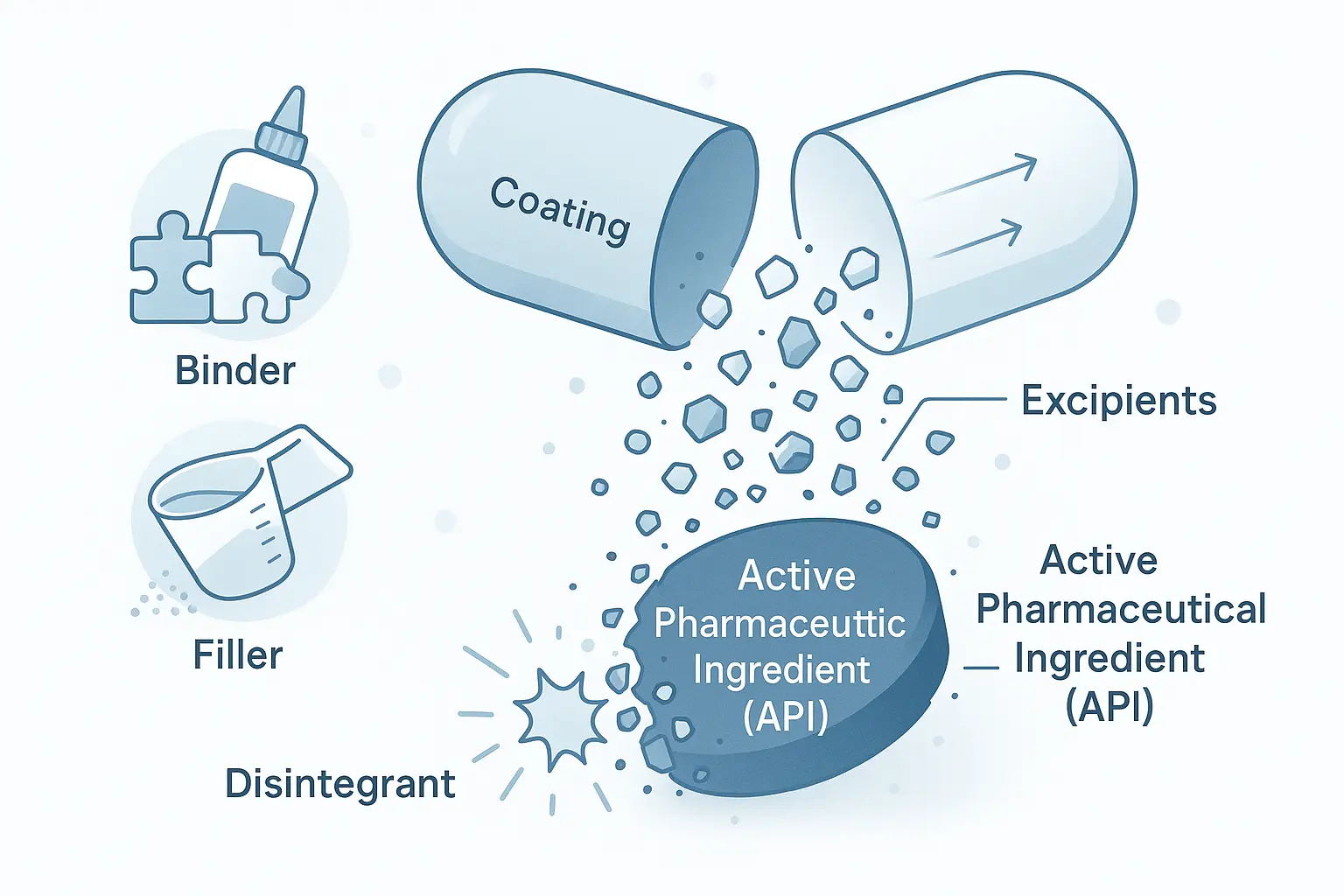
“An excipient is any element of a medicinal product that is not an active ingredient. Adjuvants, stabilizers, antimicrobial preservatives, diluents, and antioxidants all fall under the category of excipients”, As defined by the British Pharmacopoeia (BP)
Characteristics of an ideal excipient
The ideal excipients must have the following characteristics:
- An excipient should be physiologically non-reactive
- It has to show physical and chemical stability.
- It has to have reasonably good physical and chemical quality & accessible commercially.
- It should fit with the drug(s)
- It has to be non-toxic and acknowledged by the FDA or other authorities as acceptable.
- Its pricing should be reasonable.
- It ought to be devoid of any contaminants and microbiological hazards.
- The excipients should not be contraindicated when used together.
- They should not affect the bioavailability of the medication.
Classification of Excipients
Based on the pattern of use and their manufacturing
- Standard Excipients
These are either non-compendial or compendial inactive components. Among others, Lactose is a diluent, and Magnesium Stearate is a lubricant. In this sense, compendial describes excipients cataloged in the pharmacopoeia.
- Mixed Excipients
This is a simple physical mix of two or more compendial or non-compendial excipients. Excipients mixed might exist in liquid or solid form. Among the many Eudragit grades, Opadry and Opadry II are examples.
- Co-processed Excipients
This kind combines two or more compendial or non-compendial excipients employing techniques including granulation, melt extrusion, spray drying, or milling. Examples include Cellactose® 80 (comprising 75% alpha-lactose monohydrate and 25% cellulose powder), StarLac® (consisting of 85% alpha-lactose monohydrate and 15% maize starch), and Ludipress (which uses three excipients: lactose as the carrier and filler, Kollidon® 30 as the binding agent, and Kollidon® CL as the disintegrant).
Based on their origin
- Animal sources
such as Lactose, Stearic acid, Gelatin, Beeswax, and Lanolin, etc.
- Vegetable sources
such as Starch, Peppermint, Guar gum, and Acacia, etc.
- Mineral sources
such as Calcium phosphate, Silica, Asbestos, Talc, Kaolin, and Paraffin, etc.
- Synthetic sources
such as Boric acid, Lactic acid, Saccharin, Polysorbates, Povidone, and Polyethylene glycols, etc.
According to the functionality
Among the several categories of excipients are Binders, Diluents/Fillers, Disintegrants, Lubricants, Glidants, Wetting agents, Solvents, Suspending agents, Emulsifiers, Antioxidants, Preservatives, Sweeteners, Stabilizers, Coating Agents, Surfactants, Coloring agents, and Flavoring agents.
Based on their chemical composition
- Organic compounds and their salts
For example, Mannitol is employed as a diluent.
- Inorganic excipients
Like iron oxide, which is used as a pigment, inorganic excipients include calcium phosphate, which acts as a filler.
- Polymeric excipients
These could be completely synthetic or from natural sources, such as Hypromellose.
| Solid Dosage Form | Semi-Solid Dosage Form | Liquid Dosage Form | Parenteral Dosage Form |
| Diluent Filler Binder Disintegrant Lubricant Glidant Surfactant Release Modifier Coloring Agent Flavor Sweetener Coating Agent | Solvent Co-solvent Semisolid Base Antioxidant Preservative Humectant Emollient Solubilizer Coloring agent Flavor/Fragrance | Solvent Co-solvent Antioxidant Preservative Buffering agent Thickening agent Suspending agent Emulsifying agent Coloring Agent Flavoring agent Sweetener | Solvent (WFI) Co-solvent Antioxidant Preservative Buffering agent Tonicity agent Stabilizer Solubilizer |
Excipients used in Pharmaceutical Formulation
Diluents/Filler/Bulking Agents
The USP defines diluents as substances added to a tablet or capsule to raise the volume or weight of the dosage form. Diluents are fillers that handle the low bulk of the drug dose by themselves. Furthermore, diluents are often known as bulking agents or fillers. By increasing cohesion, helping in flow, supporting direct compression production, and changing the tablet’s thickness or weight, they also help to improve tablet characteristics. For tablets with modest weights, diluents are essential excipients.
Example:
- Microcrystalline Cellulose [brand names: Avicel 101, 102, 200; Celex, MCC Sanaq]
- Silicified Microcrystalline Cellulose
- Lactose [ Anhydrous Lactose, Lactose Monohydrate, Spray-Dried Lactose]
- Mannitol [preferred for chewable tablets]
- Starch [Pregelatinized Starch, Maize Starch]
- Dibasic Calcium Phosphate, Dibasic Calcium Phosphate Dihydrate, Tribasic Calcium Phosphate
- Calcium Carbonate
- Calcium Sulfate
- Magnesium Oxide
- Sodium Chloride
You can dive deeper into Fillers: Pharmaceutical Fillers: 5 Critical Reasons to Choose High-Quality Pharmaceutical Fillers
Binders
USP defines binders for tablets and capsules as materials included in formulations helping to compact powder into granules when mixed with a granulating liquid, such as water, hydroalcoholic solutions, or other solvents.
Introduced either in their dry condition or as liquids during the wet granulation process, tablet binders—also known as binding agents—are substances that create granules or encourage cohesive compacts for tablets being compressed directly. The powdered materials, known as binders or granulators, are made cohesive by employing binders.
By creating granules of the appropriate size and strength, they help to improve the flow properties and help to increase the cohesiveness of the tablet mixture, so guaranteeing that the tablet stays whole after compression.
Example:
- Povidone or polyvinylpyrrolidone has several grades: Povidone K-17, K-30, K-60, and K-90 (trademarked as Kollidon®).
- Copovidone
- corn starch
- Pregelatinized starch—known commercially as STARCH 1500
- Hypromellose, also known as hydroxypropyl methylcellulose (HPMC)
- Hydroxyethyl Cellulose
- Hydroxypropyl Cellulose
- PEG (Polyethylene Glycol).
- Low-substituted hydroxypropyl Cellulose
- Hydroxypropyl Starch
- Methylcellulose.
You can dive deep into binders: Pharmaceutical Binders: The Powerful Role and 5 Key Classifications Explained
Disintegrants
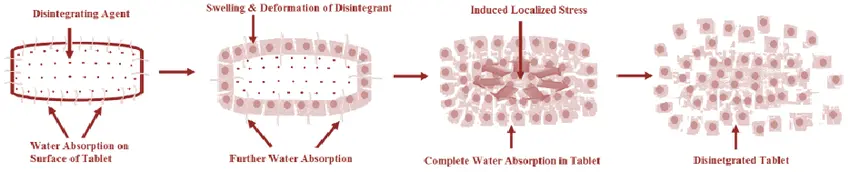
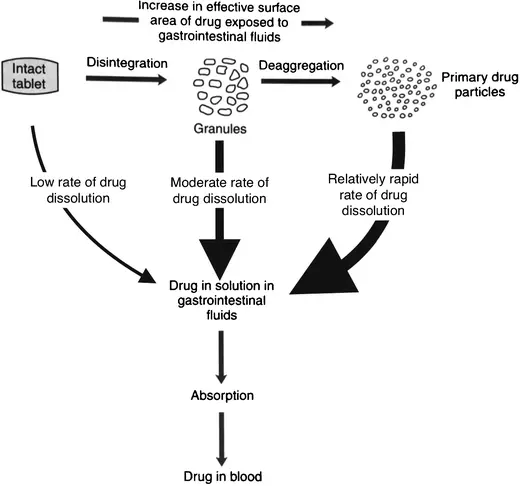
A disintegrant is a compound or a mix of substances added to a tablet to help it break down or disintegrate into tiny parts, hence enabling a pharmacological component to dissolve more quickly. The USP defines disintegrants as functional ingredients in formulations used to hasten disintegration into smaller pieces and to increase the dissolving rate of a pharmacological component. Disintegrants absorb the liquid and start to expand, disintegrate, or create gels when they touch water or digestive fluids in the stomach or intestines. The tablet structure breaks apart and disintegrates during this process, therefore increasing the surface area that helps the medication component to dissolve more.
Mechanism of Action: Disintegrants work in several ways. These are:
Swelling:
Many disintegrants, including microcrystalline cellulose and starch, absorb water and swell. Internal pressure in the tablet caused by this enlargement causes it to disintegrate. Factors including the disintegrant’s chemical composition and the tablet’s porosity determine the degree of swelling.
Wicking/Capillary Action
Disintegrants can help liquid enter the tablet, increasing the likelihood of the tablet breaking apart. Wicking action disintegrants include croscarmellose sodium and crospovidone.
Examples:
- Crospovidone, marketed as Kollidon CL, is used at concentrations ranging from 2% to 5%.
- Croscarmellose Sodium, known by the trade names Ac-Di-Sol and Primellose, is typically found in capsule formulations at 10% to 25% and in tablets at 0.5% to 5.0%.
- Low-substituted hydroxypropyl Cellulose is also utilized, alongside Sodium Starch Glycolate (which is known commercially as Primogel and Explotab) in concentrations from 2% to 8%, with the ideal amount being around 4%, although 2% is adequate in several circumstances.
- Chitosan Hydrochloride, Corn Starch, and Pregelatinized Starch are included as well, along with Calcium Alginate and Calcium Sodium Alginate, which should be kept below 10%.
- Microcrystalline Cellulose is used at levels between 5% and 15%
- Hydroxypropyl Starch
- Methylcellulose can be found at concentrations of 2.0% to 10.0%.
- Starch is typically used at 3% to 25% w/w.
- Pregelatinized Starch appears at levels of 5% to 10%.
You can dive deeper into disintegrants: Essential Guide to Pharmaceutical Disintegrants: Fast, Effective, and Easy to Understand
Lubricants
Safe, non-toxic substances with no pharmacological action, lubricants are the part of the formulation to stop tablet materials from sticking to the surfaces of the dies and punches, lower inter-particle friction, help to remove tablets from the die cavity, and maybe enhance the flow rate of the tablet granulation.
According to the USP, lubricants are usually used to lower frictional forces between particles and between the particles and the metal surfaces of manufacturing equipment, like tablet punches and dies, used in the fabrication of solid dosage forms. Liquid lubricants could be incorporated into the matrix of the tablet granules before compression.
Mechanism of Lubricants:
Boundary Lubrication
Mechanism: This is the most typical mechanism for solid lubricants employed in tablets. Usually, low shear strength, fine powder, and lubricant particles adsorb onto the surfaces of the granules and the die walls of the tablet press. This creates a thin film or boundary layer blocking direct contact between the solid surfaces. Applied force during compression and ejection concentrates the shear stress inside this easily shearable lubricant layer, hence enabling the tablet to move smoothly with less friction.
Anti-Adherent Action:
Mechanism: Lubricants also help to stop the tablet material from clinging to the punch faces of the tablet press. This pertains to boundary lubrication, in which the lubricant film on the granule surface lowers the adhesion forces between the tablet and the metal punch.
Glidant Action (Indirect Lubrication Effect):
Mechanism: Although mostly employed to enhance powder flow during processing before compression, some lubricants can indirectly support lubrication during compression by lowering interparticulate friction inside the powder bed. This enables a more consistent die fill and maybe less friction against the die walls.
Types & Example:
Water-Insoluble Lubricants:
These lubricants are hydrophobic and do not easily dissolve in water. Their main purpose is to create a physical barrier (boundary lubrication) between the tablet press equipment and the tablet parts. Although they are good at lowering friction, their hydrophobic character can occasionally impede medication solubility and tablet disintegration.
Example: Magnesium Stearate, Calcium Stearate, Stearic Acid, Glyceryl Behenate, Talc (Hydrated Magnesium Silicate), Colloidal Silicon Dioxide (Cab-O-Sil, Aerosil).
Water-Soluble (Hydrophilic) Lubricants:
Generally preferable when fast tablet disintegration and medication dissolution are sought; these lubricants dissolve in water and are less prone to form a hydrophobic barrier. Often needing greater concentrations, they may not be as effective as water-insoluble lubricants in lowering friction and avoiding sticking.
Example: Polyethylene Glycols (PEGs), Sodium Stearyl Fumarate, Sodium Lauryl Sulfate (SLS), Magnesium Lauryl Sulfate, polysorbate 20, and polysorbate 80.
Glidant / Anticaking Agent
Non-toxic and pharmacologically inactive, a Glidant is an agent that enhances the flow characteristics of tablet granulation or powdered materials by reducing particle friction and cohesiveness. Usually, these substances are added in their dry form during the lubrication phase before compression.
Glidants and anticaking chemicals, as defined by the USP, increase powder flow and lower clumping possibly resulting from bulk storage. Glidants and anticaking agents also help to reduce the likelihood of bridging during powder hopper discharge and powder processing.
Example:
Hydrophobic Colloidal Silica, Magnesium Trisilicate, Magnesium Silicate, Sodium Stearate, Calcium Silicate, Tribasic Calcium Phosphate, Talc, Colloidal Silicon Dioxide (commercial name: Aerosil 200/ Cab-o-sil).
Coloring Agents / Colorant
Non-active chemicals added in dosage forms called coloring agents give them a unique appearance, which can help distinguish a product from others with comparable physical characteristics or, in some cases, to protect components of the dosage form that are light-sensitive. Moreover, coloring chemicals are referred to as colorants.
Coloring agents fall into different categories:
Dyes: Coloring substances that dissolve in water,
Lakes: Insoluble forms of a dye created by its permanent adsorption onto a hydrous metal oxide,
Inorganic pigments: Materials like titanium dioxide or iron oxides, and
Natural colorants: Colored compounds that are not classified as dyes, such as riboflavin.
Example:
FD & C Red #40 /Allura Red AC, FD & C Blue #1 /Brilliant Blue FCF, FD & C Red #3 /Erythrosine, Fast Green FCF, Green S (Lissamine Green), FD & C Red #30 /Helendon Pink, FD & C Blue #2 /Indigo Carmine, FD & C Red, Patent Blue, Iron Oxide Yellow, Quinoline Yellow, Caramel, Ferric Oxide, Titanium Dioxide, Ferrosoferric Oxide, Aluminum Oxide, FD & C Red.
Flavoring agent and Fragrance
The USP defines a flavor as either a single chemical or a combination of natural or synthetic chemicals that, when consumed or inhaled, produces a taste or olfactory impression. While fragrances are meant solely for external usage and are enjoyed simply for their aroma, flavoring chemicals are eaten and enjoyed via both taste and smell. Usually, flavors are necessary as excipients in chewable tablets, orally disintegrating tablets, dispersible tablets, oral solutions, and oral suspensions to mask undesirable tastes and odors, therefore enhancing the enjoyment of the product and supporting patient compliance.
Example:
Vanillin, peppermint flavor powder, strawberry flavor powder, orange flavor powder, lemon flavor powder, orange essence, eucalyptus oil, sodium succinate, almond oil, menthol, peppermint oil, rose oil, rose water, stronger.
Sweetener or Sweetening agent
Ingredients used to mask bad tastes and improve the sweetness of oral dose forms are called sweeteners. They cling to the taste buds on the tongue that sense sweetness. In chewable pills, lozenges, orally disintegrating tablets, dispersible tablets, oral solutions, emulsions, and oral suspensions, sweeteners are a crucial excipient.
Example:
Aspartame, Dextrose, Fructose, Maltose, Mannitol, Saccharin, Sorbitol, Xylitol, Sucralose, Saccharin Sodium, & Sucrose.
Tablet Formulation Case: Paracetamol 500 mg Immediate-Release Tablet
Let’s formulate a Paracetamol (Acetaminophen) 500 mg tablet — one of the most widely used OTC analgesics — focusing on the functionality of each excipient.
Target Profile
- Dosage form: Immediate-release tablet
- Route: Oral
- Dose: 500 mg
- Desired attributes: Rapid disintegration, easy swallowing, acceptable taste, cost-effectiveness
| Component | Amount (mg/tab) | Function |
|---|---|---|
| Paracetamol | 500.0 | Active Pharmaceutical Ingredient (API) |
| Microcrystalline Cellulose (Avicel PH 102) | 100.0 | Filler & dry binder; provides compactability |
| Starch (Maize or Pregelatinized) | 50.0 | Dilluent |
| Povidone (PVP K30) | 10.0 | Binder (wet granulation) |
| Croscarmellose Sodium | 15.0 | superdisintegrant |
| Magnesium Stearate | 5.0 | Disintegrant & filler promote breakdown in GI |
| Talc | 5.0 | Glidant & anti-adherent |
| Total | 685.0 mg | Disintegrant & filler; promote breakdown in GI |
Explanation of Excipient Roles
- Microcrystalline Cellulose gives excellent compressibility, ensuring the tablet can be made with less pressure and still be strong.
- Croscarmellose Sodium helps the tablet break apart quickly in the stomach (essential for fast onset).
- Povidone creates a strong internal matrix when granulated, ensuring uniformity and durability.
- Magnesium Stearate & Talc ensure smooth tablet manufacturing, reducing friction and sticking to dies.
Key Considerations
- Disintegration Time: <15 minutes (as per the pharmacopeia)
- Hardness: 6-15 kg/cm² (ensures durability without affecting release)
- Friability: <1% (checks resistance to chipping/breaking)
- Weight Variation: ±5% (critical for dose accuracy)
💡 Formulators often adjust disintegrant and binder ratios based on granule flowability, compressibility, and dissolution testing. Even a 0.1%-0.5% shift in magnesium stearate can alter tablet hardness or dissolution profile.
conclusion
Pharmaceutical excipients play a vital role far beyond simply serving as inactive ingredients—they are key to the stability, effectiveness, and overall success of pharmaceutical formulations. In this first episode, we’ve laid the foundation by exploring excipients’ general functions and significance in drug development. However, this is just the beginning. In the next article, we’ll dive deeper into the specific categories of excipients, their selection criteria, and how they influence various dosage forms. Stay tuned as we continue uncovering the science behind these essential components in pharmaceutical formulation.
References


