Excipients are inactive substances used as carriers for the active ingredients of a medication. At the same time, they are often referred to as “inactive” excipients, but they play a critical role in the formulation, manufacturing, and performance of pharmaceutical products. They influence the drug’s delivery, stability, bioavailability, and patient acceptability. The performance of excipients is determined by their ability to perform specific functions without compromising the therapeutic efficacy of the active pharmaceutical ingredient (API). This article explores the functions, evaluation, challenges, and advancements in pharmaceutical excipient performance.
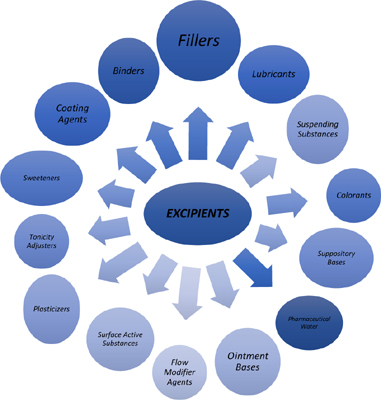
Functions of Excipients
Excipients serve various functions in pharmaceutical formulations, each contributing to the overall quality and effectiveness of the final product. Below are the key roles excipients play:
- Binders:
Binders are essential in tablet formulations, as they hold the ingredients together, ensuring that the tablet remains intact after compression. Common binders include microcrystalline cellulose, polyvinylpyrrolidone (PVP), and starch. Without binders, tablets would crumble during handling or transportation, rendering them ineffective. - Fillers/Diluents:
Fillers, also known as diluents, provide bulk to the formulation, making it easier to handle and administer. This is particularly important for drugs with very small doses, where the active ingredient alone would be insufficient to form a tablet. Examples of fillers include lactose, calcium phosphate, and mannitol. Fillers also ensure uniformity in drug distribution within the tablet.
- Disintegrants:
Disintegrants are crucial for ensuring that the tablet breaks down quickly after administration, allowing the active ingredient to be released and absorbed by the body. Common disintegrants include croscarmellose sodium, sodium starch glycolate, and crospovidone. The efficiency of disintegrants directly impacts the drug’s bioavailability and therapeutic effect. - Lubricants and Glidants:
Lubricants and glidants are used to improve the flow of powder blends during the manufacturing process. Lubricants, such as magnesium stearate and stearic acid, prevent the powder from sticking to the equipment, while glidants, like colloidal silicon dioxide, enhance the flow properties of the powder. These excipients ensure smooth tablet production and reduce manufacturing defects. - Preservatives:
Preservatives are added to pharmaceutical formulations to prevent microbial growth and extend the shelf life of the product. Common preservatives include benzalkonium chloride, methylparaben, and propylparaben. They are particularly important in liquid and semi-solid formulations, such as syrups and creams, where the risk of contamination is higher. - Flavoring Agents:
Flavoring agents are used to improve the taste and palatability of oral medications, especially for pediatric and geriatric patients. Examples include sucralose, menthol, and fruit flavors. By masking the bitter or unpleasant taste of the API, flavoring agents enhance patient compliance and adherence to treatment regimens.
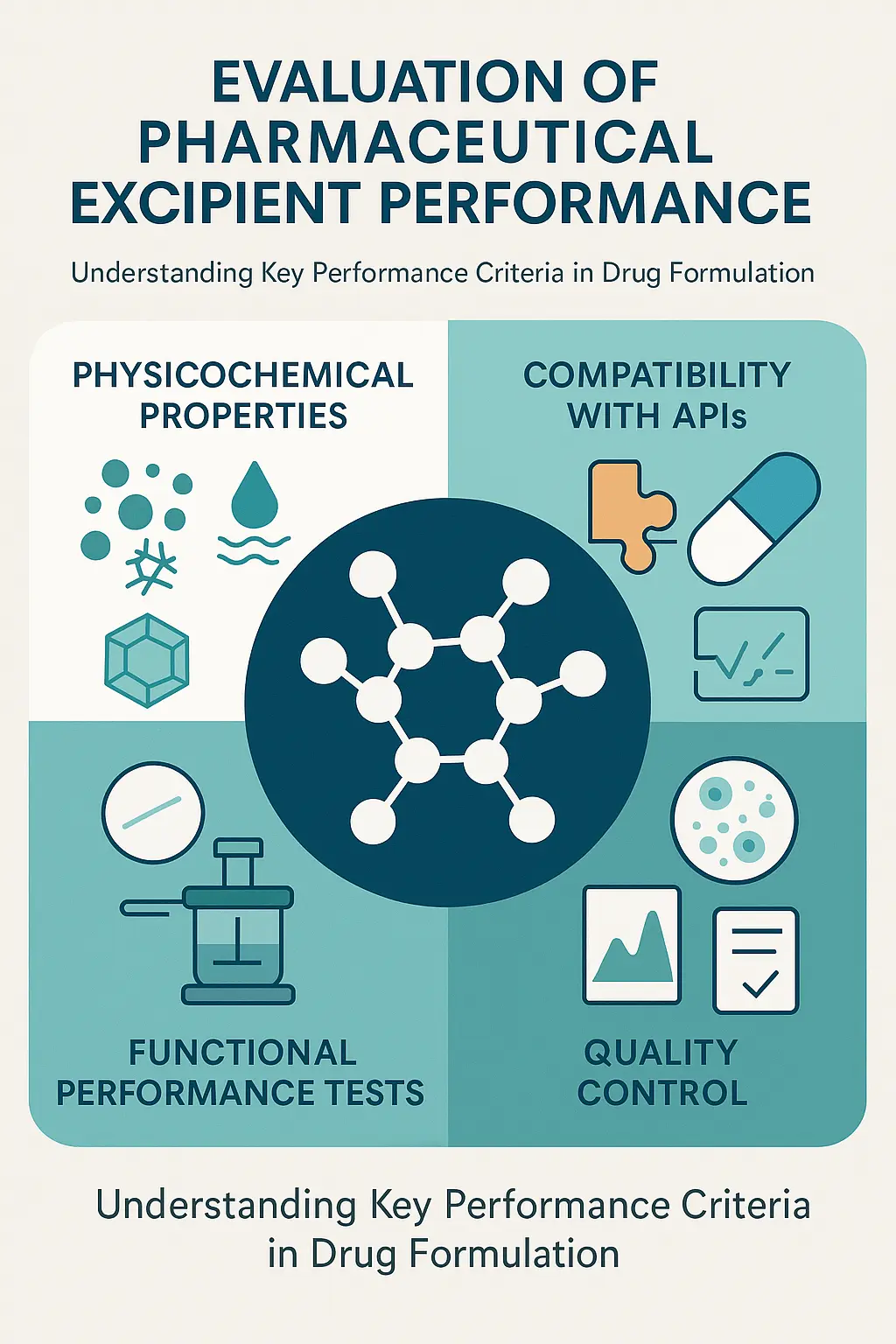
Evaluation of Pharmaceutical Excipient Performance
The performance of excipients is evaluated through a series of tests and assessments to ensure they meet the required standards for safety, efficacy, and functionality. Below are the key aspects of excipient evaluation:
- Physicochemical Properties:
The physicochemical properties of excipients, such as particle size, shape, hygroscopicity, and polymorphism, are critical to their performance. For example, particle size can affect the flowability of powders, while hygroscopicity (the ability to absorb moisture) can impact the stability of the formulation. Polymorphism, or the existence of multiple crystalline forms, can influence the solubility and bioavailability of the API. - Compatibility with APIs:
Excipients must be compatible with the active pharmaceutical ingredient to ensure the drug’s efficacy and safety. Incompatibility can lead to chemical reactions, degradation of the API, or changes in the drug’s release profile. Compatibility studies are conducted using techniques such as differential scanning calorimetry (DSC) and Fourier-transform infrared spectroscopy (FTIR). - Functional Performance Tests:
Functional performance tests evaluate the specific roles of excipients in the formulation. For example, binders are tested for their binding strength, disintegrants for their disintegration efficiency, and lubricants for their lubrication properties. These tests ensure that the excipients perform as intended in the final product. - Quality Control:
Rigorous quality control tests are conducted to ensure that excipients meet the required standards for safety and efficacy. These tests include impurity profiling, microbial testing, and stability testing. Quality control is essential to prevent contamination and ensure batch-to-batch consistency.
Challenges of Pharmaceutical Excipient Performance
Despite their importance, excipients present several challenges in pharmaceutical formulation and manufacturing. Below are some of the key challenges:
- Variability in Natural Excipients:
Natural excipients, such as starch and cellulose, are derived from biological sources and can exhibit variability in their properties. This variability can affect the consistency of the final product, leading to issues such as differences in tablet hardness or dissolution rates.
Natural excipients like starch and cellulose are derived from biological sources, leading to inherent variability due to factors such as:
- Source Diversity: Different plant species or parts used.
- Growing Conditions: Soil quality, climate, and agricultural practices.
- Processing Methods: Extraction and purification techniques.
- This variability can impact critical quality attributes (CQAs) of pharmaceutical products
- Dissolution Rates: Variability may affect how quickly the drug is released
- Overall Product Quality: Batch-to-batch inconsistencies can compromise efficacy and safety
- Tablet Hardness: Inconsistent excipient properties can lead to variations in tablet strength.
- Regulatory Requirements: Excipients must meet stringent regulatory requirements to ensure they are safe for use in pharmaceutical products. Regulatory agencies, such as the U.S. Food and Drug Administration (FDA) and the European Medicines Agency (EMA), require extensive documentation and testing to approve excipients. Meeting these requirements can be time-consuming and costly.
- Formulation Complexity: The development of novel drug delivery systems, such as controlled-release formulations and nanoparticle-based delivery systems, often requires the use of sophisticated excipients. These excipients must be carefully selected and tested to ensure they perform as intended in the complex formulation.
Ideal Pharmaceutical excipient properties
1. Inertness (Non-reactivity)
- Does not interact with the active pharmaceutical ingredient (API) or other excipients.
- Chemically and physically stable under formulation and storage conditions.
2. Biocompatibility and Safety
- Non-toxic, non-irritating, and safe for the intended route of administration (oral, topical, injectable, etc.).
- Approved by regulatory agencies like FDA or EMA.
3. Functionality
- Performs its intended role effectively, such as:
- Binder (for tablet cohesion)
- Filler/Diluent (to add bulk)
- Disintegrant (to aid breakdown in the body)
- Lubricant/Glidant (to improve flow and prevent sticking)
- Coating agent (for taste-masking or controlled release)
4. Consistency and Reproducibility
- Minimal batch-to-batch variability to ensure uniform performance.
- Especially critical for natural excipients (e.g., starch, cellulose) where source variation can impact product quality.
5. Good Processability
- Compatible with manufacturing techniques (e.g., direct compression, wet granulation).
- Possesses good flow properties, compressibility, and blending capacity.
6. Stability
- Physically and chemically stable during manufacturing, storage, and shelf life.
- Does not promote degradation of the API.
7. Acceptable Organoleptic Properties
- Neutral taste, odor, and appearance (especially important for oral formulations).
- Should not negatively impact patient compliance.
8. Regulatory Acceptance
- Listed in pharmacopoeias (e.g., USP, EP) or previously used in approved products.
- Comprehensive documentation and safety data are available.
9. Economical and Available
- Cost-effective at commercial scale.
- Widely available from reliable suppliers with strong quality control systems.
10. Environmental and Sustainability Profile
- Preference for excipients with a low environmental impact and sourced sustainably (growing in importance).
Advancements and Innovations in Excipient Science
The field of excipient science is continuously evolving, with ongoing research and innovation aimed at improving the performance and functionality of excipients. Below are some of the key advancements:
- Novel Excipients:
Researchers are developing novel excipients with enhanced functionalities, such as modified release profiles and targeted delivery mechanisms. For example, pH-sensitive polymers can be used to create enteric-coated tablets that release the drug in the intestine rather than the stomach. - Co-processed Excipients:
Co-processed excipients are created by combining two or more excipients to enhance their performance. For example, Ludipress is a co-processed excipient that combines lactose, PVP, and crospovidone to improve flowability, compressibility, and disintegration. Co-processed excipients simplify the formulation process and reduce the number of ingredients needed. - Green Excipients:
With increasing focus on sustainability, researchers are developing green excipients that are derived from renewable sources and have a lower environmental impact. Examples include plant-based polymers and biodegradable materials. These excipients not only reduce the environmental footprint of pharmaceutical manufacturing but also align with the growing demand for eco-friendly products.
Conclusion
Excipient performance is a critical aspect of pharmaceutical formulation that directly impacts the quality, efficacy, and safety of the final drug product. From binders and fillers to disintegrants and lubricants, excipients play a vital role in ensuring that medications are effective, stable, and patient-friendly. However, challenges such as variability in natural excipients, regulatory requirements, and formulation complexity must be addressed to ensure consistent performance.
Advancements in excipient science, including the development of novel excipients, co-processed excipients, and green excipients, are driving innovation in the pharmaceutical industry. These advancements are not only improving drug delivery systems but also contributing to the development of more sustainable and environmentally friendly products.
As the pharmaceutical industry continues to evolve, the importance of excipient performance will only grow. Continuous research and innovation in excipient science are essential for advancing drug delivery systems, improving patient outcomes, and ensuring the safety and efficacy of pharmaceutical products.
References:
USP 〈1059〉 EXCIPIENT PERFORMANCE



