Introduction
Pharmaceutical binders, also known as binding agents, are substances added to a drug product formula to form granules, blend for final tablet form, or to be filled in hard gelatin capsule shells. They play an important role in ensuring the cohesion and plasticity of the blend powder mixture, which enhances the tableting process (blend integrity) and decreases the risk of tablet breakage during manufacture, shipping, or transportation.
Importance and main uses
They are employed to impart cohesiveness to the granules. They are also essential for maintaining the integrity of the tablet. These materials are added to the tablet formulation to impart plasticity as well as increases inter-particulate bonding strength in the tablet that ensure the tablet remains intact after compression. They help in increasing the particle size, improving flowability, reducing bulk volume, and minimizing dust content. The proper amount is crucial for ensuring that the tablet remains intact until it reaches its target.
To hold various powders together to form a tablet, it is added either in a dry mix or mixed with granulating liquid and forms a matrix with fillers and the drug embedded in it. On drying, solid drug-binding agents form glue which holds the particles together, the wet drug-binding agents are the most important ingredient in the wet granulation process; most of them are hydrophilic & most time soluble in water.
Main types and classifications
They can be classified based on their origin and application in the manufacturing process:
A- Origin-based classification:
- Natural: These are derived from natural sources and are less toxic and biodegradable. Examples include starch, pregelatinized starch, sodium alginate, pullulan, and gelatin. They are available and low-cost.
- Synthetic/Semi-Synthetic: These are widely used and require a low amount in formulation. Examples include polyvinyl pyrrolidone (PVP); which has excellent binding properties; methylcellulose, hydroxypropyl methylcellulose (HPMC), which provides good binding and film-forming properties; dextrin and polyethylene glycol (PEG), used for their excellent binding and lubricating properties.
- Saccharides and Their Derivatives: These include disaccharides such as lactose and sucrose, and polysaccharides like cellulose and modified cellulose.
- Others with sustained release properties: Fatty alcohols, Fat and Waxes, Hydrated Rizinius Oil, Stearic Acid
B- According to the shape:
- Linear: Example – Amylose, cellulose, pectin
- Branched: Short branch – Example – Xylan, xanthan
- Branch on branch–Example-Amylopectin, gum arabic
C- According to the charge:
- Non-ionic: Example – Guar gum, xanthan gum, dextrin
- Anionic: Example – Alginic acid, pectin, karaya gum
- Cationic: Example – Chitosan, chitin, cationic guar gum
- Amphoteric: Example – Carboxymethyl chitosan, N-Hydroxyldicarboxyethyl chitosan
- Hydrophobic: Example–Cetylhydroxyethyl cellulose, polyquaternium
D- According to the monomeric units present in the chemical structure:
- Homoglycan: Example–Amylose, arabinose, cellulose
- Diheteroglycan: Example–Algins, carrageenan, galactomannans
- Triheteroglycans: Example – Arabinoxylans, gellan gum, xanthan gum
- Tetrahetero glycans: Example–Gum arabic, psyllium seed gum
- Pentahetero glycans: Example–Gum ghatti, gum tragacanth
E- Manufacturing-based applications classifications:
They can be used in different stages of the manufacturing process:
1. Dry Binders: These are excipients that are used in the manufacture of tablets or capsules, often prepared by direct compression. Under their compaction properties, they can form densified compacts in the presence of the bulk drug substance and other excipients (e.g., disintegrants and lubricants) without the need for prior dry or wet granulation.
The primary physical properties relevant to dry drug-binding agents are those that can have a direct effect on the material and formulation performance. These include particle size and size distribution, particle shape, bulk density, tapped density, true density, specific surface area, crystallinity, moisture content, powder flow, solubility, crystal form, and compaction properties.
These materials comprise a diverse group of materials that include inorganics (e.g., dibasic calcium phosphate), single-component organic materials (e.g., mannitol), excipient blends, and co-processed excipients such as silicified microcrystalline or complex organics (e.g., microcrystalline cellulose or pregelatinized starch). They may be soluble or insoluble in water, and they may be neutral, acidic, or alkaline in nature. These chemical properties can have a positive or negative effect on the drug substance’s physical or chemical stability and performance.
Appropriate selection of these materials with desirable physical and chemical properties can enhance the physical and chemical stability as well as the performance of the drug substance and dosage form. The detailed composition may be important because its function can be influenced by the presence of minor components that are essential for proper performance. Pharmacists may find it necessary to control the presence of impurities (e.g., elemental impurities or peroxides) to ensure adequate dosage form stability and performance.
2. Wet Binders: These materials are incorporated into formulations to agglomerate powder to form granules using a granulating fluid such as water, hydroalcoholic mixtures, or other solvents. They include natural materials such as sugars and starch, and semisynthetic and synthetic polymers such as modified cellulose and povidone. The typical use level is between 2% and 20%, depending on physicochemical properties, formulation components, and composition. It may be incorporated either dissolved in the granulating fluid or dry blended with the other powders to be wet granulated.
Important physical properties include solubility in the granulating fluid, spreadability on the powder substrate, the ability to cause the powders to adhere and agglomerate, and the ability to provide plasticity to the granules. Homogeneous incorporation into a dry blend also depends on particle size distribution and morphology. High viscosity may reduce spreadability. Viscosity is dependent on polymer structure and molecular weight. Excessive levels, especially high viscosity grades, may form gels and retard drug dissolution.
They are also a diverse range of materials, usually polymeric. The chemical nature of polymers, including monomer sequence, functional groups, degree of substitution, and branching, can influence the complex interactions during granulation.
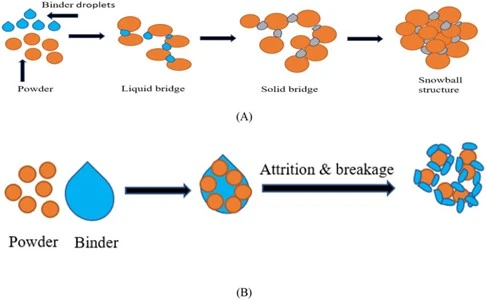
The adhesive properties of binding solutions facilitate the agglomeration of powders into granules. It may modify interfacial properties of poorly water-soluble drug substances, improving wettability and facilitating subsequent dissolution from the tablet. During drying, they produce solid bridges between the powder particles, yielding granules with improved properties such as flow, handling, mechanical strength, resistance to segregation, reduced dustiness, and compatibility.
They are also used to manufacture solid oral dosage forms, including tablets, capsules, granules, pills, and pellets. Properties that may be important for excipient performance in a dosage form include viscosity. Particle size distribution may be important when being incorporated by dry blending.
Conclusion
Pharmaceutical drug-binding agents are vital components in the formulation of tablets and other solid dosage forms. They ensure the cohesion and plasticity of the powder mixture, which is essential for the manufacturing process and the integrity of the final product. Selecting the appropriate binding agent is critical to achieving the desired release profile and ensuring the stability of the tablet.
References:
- USP 〈1059〉 EXCIPIENT PERFORMANCE
- Quick Look: The Role of Functional Excipients
- Tablet Binders: Types & Examples with Concentration
- Binders Need and used in Solid Formulation, Vikranti W. Koli, Manohar D. Kengar, Amruta B. Kamble, Suraj B. Kumbhar, Rahul P. Jadhav


