The objective of this article
The objective of this article is to concentrate on the importance and expectations in production scheduling in pharmaceuticals and will also give a briefing on the process flow from the start till the end, i.e., starting from the forecast till the order fulfillment. This article will provide a holistic view of the drawbacks of scheduling. In this modern world, it has become very challenging to run a smooth supply chain operation. To carry out a smooth run, it is very much essential to ensure a smooth production planning based on the forecast. The importance of ERP is also enlightened in this article.
Introduction to production scheduling
Production scheduling in pharmaceutical operations is a mechanism to carry out the planning of products starting from the forecast till the completion of the order quantity
Why is scheduling necessary?
Scheduling is required to complete the order in required time frame. Scheduling cannot be performed single handedly as it requires other networking to perform smooth operation without any hindrance. This networking chain for scheduling is called supply chain operations.
The following departments come under the domain of supply chain operations.
- Planning department for existing products
- Production department for the manufacturing ingredients
- Procurement department for the packaging items
- Procurement of controlled substances
- Procurement department for the general items
- Resources allocators
- Raw Materials Warehousing
- Packaging material Warehousing
- Planning Department for new products
- The distributor
Other than these departments, there are roles from other important departments which is not ignorable to complete the order.
- Dispensing
- Production Manufacturing
- Production Packaging
- Quality Control
- Quality Assurance
- Quarantine store
- Finished goods department
These all sections are having importance in production scheduling in pharmaceuticals. Missing of any section might lead to any big undesirable surprise and may damage the prestige of company.
How does the production scheduling drive?
The main driver of production scheduling is the forecast. Forecast plays an important role in production planning. There is a case study on forecasting which can be explored further to get a better understanding: “Demand forecasting in pharmaceutical supply chains: A case study.”
The sales and marketing department shares the forecast with the Supply chain department (Planning operations).
Sales and Marketing department develops the data based on the following attributes:
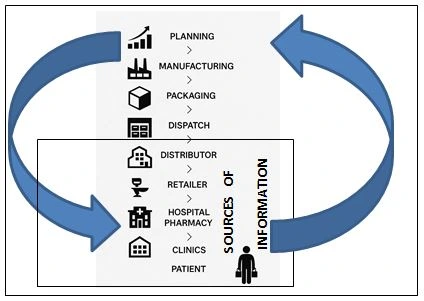
FIGURE 1: Source of information to perform production scheduling
- Customer needs are driven by the product sales data
- Purchase orders of product within last three months but this duration may vary company to company some companies take purchase data of three month and some companies take data of even one month and generate forecast.
There is an example of a forecast,
A product name ABC is an antidiabetic drug containing metformin and sitagliptin as active pharmaceutical ingredients. Consider the data for forecasting,
Product Name: ABC
Let’s suppose the following hypothetical data for January 2025 of a XYZ Pharmaceutical located in a modern world.
| Supply cities | Distributor Order | Pharmacy Store Orders | Sales Data of a Hospital Pharmacy | Cumulative Sales Data |
| City A | 10000 packs | 2000 packs | 200 packs | 3200 packs |
| City B | 1000 packs | 4000 packs | 1800 packs | 6800 packs |
| City C | 5000 packs | 3000 packs | 5000 packs | 13000 packs |
| City D | 3000 packs | 1000 packs | 4000 packs | 8000 packs |
The data conclude that XYZ pharmaceutical has sold 31000 packs in four cities of a modern country. The marketing department predicts with this data that this is an ant diabetic drug that is not an OTC. The customers of these products are regular, and they won’t like to miss the dose to prevent their normal diabetic level excursions. Although one month of data is not sufficient for the validated forecast, but subjected to the qualitative nature of this drug, the marketing department can predict a forecast of more than 31000 packs to keep the safe stock level and the survival of the product in the market. The marketing department will predict a forecast of around 40000 packs.
Role of the Planning Department in the execution of the scheduling of a product
In pharmaceutical operations, some manufacturers have thousands of SKUs. All these SKUs are controllable if a good scheduling system is in place. A good planner must have the data for production line capacities. Sometimes the forecast of product does not meet the available capacity, so companies plan extended hours for the completion of orders. The planning department schedules the purchasing of materials and forwards the material requirements to procurement to arrange them on the given timelines. As per the arrivals expected, planning had already scheduled the quality testing, material dispensing, and production. There is a tool named, Gantt chart that can be utilized to visualize the presentation of scheduling.
The example of a Gantt chart in bottleneck situations is explained below.
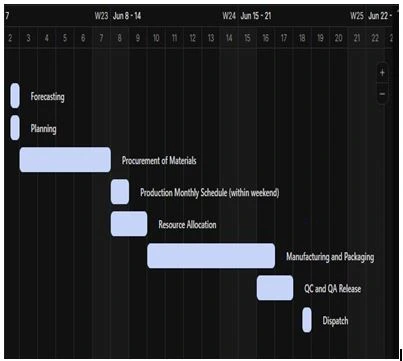
Gantt chart Review:
Visualization of activities in the above Gantt chart from the 2nd of June till the 18th of June, forecasting till dispatch, and the duration of each activity is clearly visible. This tool effectively monitors the progress of each task and helps with timely order fulfillment.
Importance of a scheduling mechanism
A good scheduling mechanism must possess the following attributes,
- Predetermined production capacities
- Predetermined Product Cycle Time
- Predetermined lead time for the arrival of required materials
- A good performance management system
- Safety stock level of APIs and excipients in store to prevent any rushed situation
- Flexibility in the planning of products as per the forecast
- A Production plan should have a leverage of around 20% unplanned downtime.
- Scheduled maintenance is considered while scheduling production
- Labor allocations must not be ignored during production scheduling
- Over forecasting or seasonal augmentation of any product must have room for scheduling
- Reduce change overs by presenting fixed repeating schedule for a product i.e. Product XYZ one batch needs to manufacture every month. A good scheduler plans three batches a month to prevent change over on monthly basis. This concept is also called campaign production of batches. This campaign run is widely adopted in pharmaceuticals which is preapproved and validated in pharmaceutical system.
- Prevent wastages i.e., keeping a good inventory of all the raw materials, and do not plan overcapacity and under capacity.
- Overcapacity scheduling will waste energy utilization due to heavy plant operations activities. Overcapacity scheduling may increase the stock levels of planned products. Overcapacity will also ignore the required maintenance of machines and will produce wear and tear on machines.
- Under capacity scheduling will waste the process hours and labor hours.
These are the most important scheduling attributes which must be adopted by supply chain operations to perform the operations smoothly.
Planning For The Purchases
There are three major types of purchases in supply chain operations.
Procurement of General Items:
This type of procurement generally covers Opex items. There is an example that in a sterile area, product manufacturing requires filters to ensure the product is free from foreign particles. Disinfectants are required to produce a clean environment in production areas.
Procurement of Raw Materials:
Raw material procurement is a complex procedure in any pharmaceutical because of strict compliance rules. In general, if we need to purchase an item that is not available from brand A so we can procure the same item from brand B.
In pharmaceuticals, procurement ensures compliance because the quality assurance department is responsible for qualifying the vendors. Ordering will be done by only the selected approved vendors list, which is validated and authorised by the quality assurance department.
Procurement is not allowed to change the source of materials. Source approval is mandatory before purchasing. In case of a new source approval, the procurement department arranges the sample of the required source and provides the sample to quality control for testing purposes. If the specifications of the material meet the quality criteria for approval, then management will ask to arrange the procurement of the material to manufacture a pilot-scale batch. If the results and stability of the product are satisfactory for the required duration, then the procurement of material for commercial production will be scheduled. In case of alternate sourcing, it is mandatory to keep in mind the regulatory guidelines.
How does the procurement of Raw materials work?
In every pharmaceutical, there is an approved recipe for each product, and that recipe contains the BOM, i.e., Bill of Materials.
A bill of materials contains the list of ingredients needed to
Procurement of Packaging Materials:
Packaging material is divided into three types of packaging.
- Primary packaging:
Packaging that comes in direct contact with the product, like tablets, is filled in an HDPE bottle.
- Secondary Packaging:
Secondary packaging contains the primary container, like an HDPE bottle is packed in a unit carton
- Tertiary Packaging:
This packaging contains multiples of packed unit cartons, like unit cartons are packed in a Master carton or a Shipper.
In pharmaceutical, it is obvious to arrange the material as per the approved specifications. Packaging materials vary from product to product and finalise at the time of product development. Procurement department arranges the packaging material as per the provided schedule.
Resource allocations
It is an essential part of production scheduling because without the available resources order fulfilment cannot be executed. It is mandatory to schedule the production by considering the availability of machines, availability of head count and availability of in direct resources like lint free dusters, cleaning disinfectants etc.
Raw and Packaging Material Warehousing
The question arises how it comes under the scheduling. The answer is when the procurement of raw or packaging material has been executed, and some of the inventory is already in place, so there must be a proper storage capacity to store the ordered material, so the scheduler will ensure that the procurement should not be over capacity or under capacity of the warehouse.
Raw and packaging warehouses are the facilities where the inventory of ingredients, packaging materials is kept with proper segregation, like active ingredients, excipients, primary packaging, secondary packaging, and tertiary packaging are palletized separately. Along with these separation warehouse always ensures to palletize released and unreleased materials separately. A warehouse always follows the FIFO (First in First Out) procedure to maintain the inventory with a good shelf life
New product planning system
In the planning of a new product scheduler receives the demand from business development to procure the material for a trial batch. The product development section provides the list of ingredients required for the trial batch of a new product. Scheduler starts sourcing of material and once arranged, provides these materials to the NPD section
The Distribution Network
In the pharmaceutical sector, there is a proper authorized distribution network responsible for dispatching the product to the required zones i.e., wholesalers and retailers, and hospital pharmacies.
Production Planning Adherence Check
Consider an example of production scheduling adherence.
| Date Scheduled | Activity | Capacity | Planned |
| 1st june, 2025 | Dispensing | 5 batches per day | 4 batches per day |
| 3rd june, 2025 | Manufacturing (Granulation) | 4 batches per day | 4 batches per day |
| 4th june, 2025 | Compression | 1.5 Million tabs per day | 2 Million Tabs per day |
| 5th june, 2025 | Coating | 4 batches per day | 2 batches per day |
| 6th june, 2025 | QC Release | 1 day for Testing | 1 day for testing |
| 7th june, 2025 | Packaging | 0.5 million packs per day | 0.1 Mn packs per day |
| 8th june, 2025 | Market Dispatch | 0.5 million packs per day | 0.5 Million packs per day |
Data revealed that scheduled activities are as per the available capacity, except for the highlighted unit operation, and this type of doable scheduled activities is called Finite capacities. Only the highlighted unit operation is over Capacity, and that is called Infinite capacity planning. To resolve the bottleneck of compression, the planner provides multiple suggestions like prioritizing the products as per the requirement in the market, extending work hours on compression, and planning a double shift on compression.
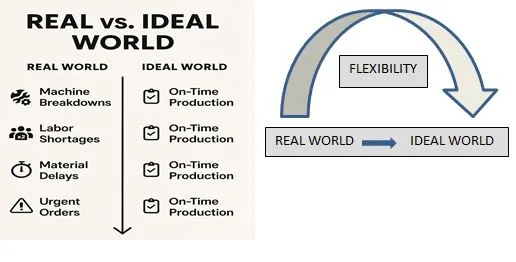
Figure 2: IDEAL VS ACTUAL SCHEDULING SCENARIO
Importance of performance management in production scheduling
Performance management is necessary to ensure the progress of the set schedule. In a performance management system, management creates KPIs, shows visualizations, identifies bottlenecks, and ensures problem-solving

Figure 3: fruitful meeting on bottlenecks and problem solving
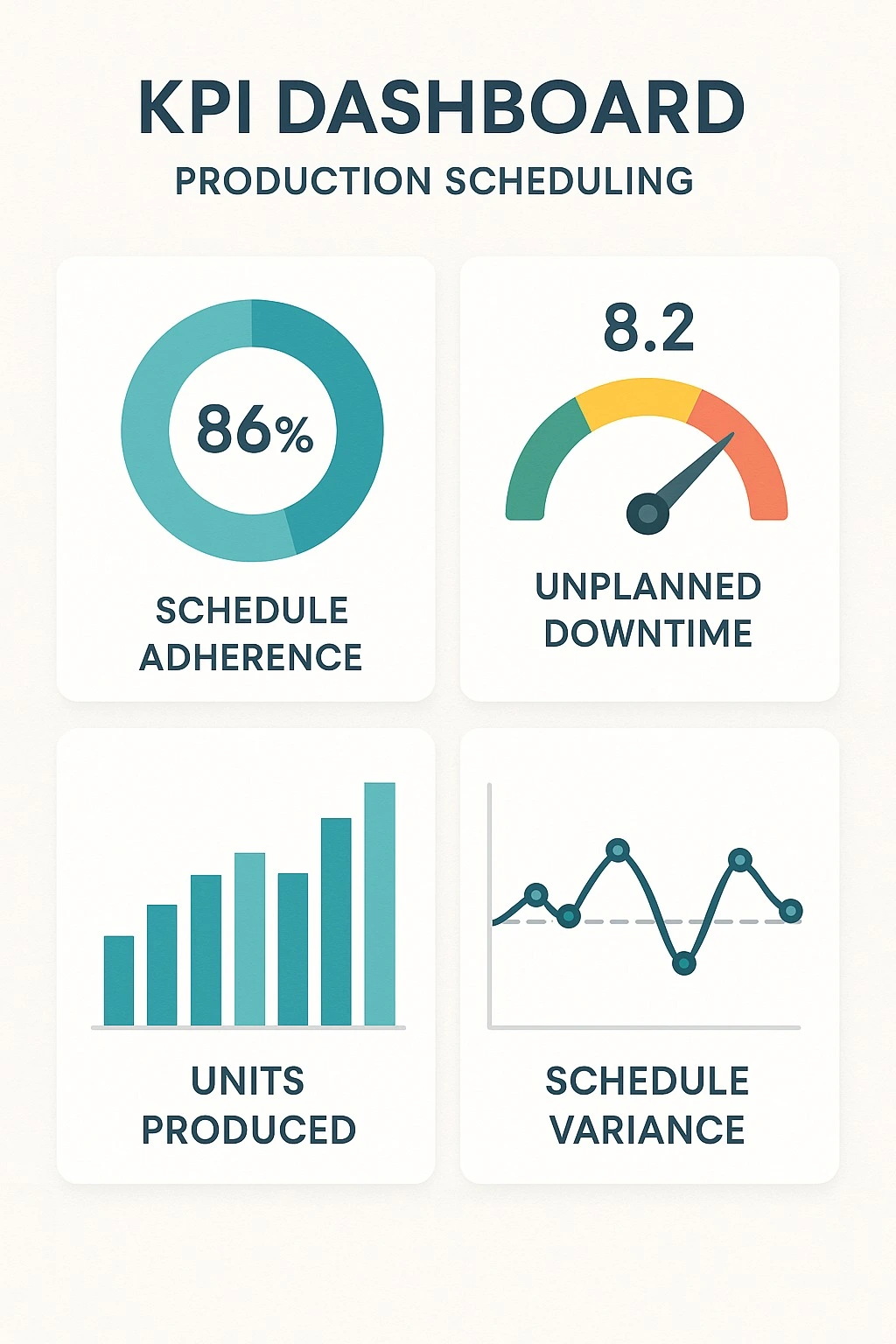
Figure 4: KPI DASHBOARD TO MONITOR PROGRESS
Common drawbacks of poor scheduling
Poor scheduling is when the plan does not consider the important aspects of scheduling, like the lead time for procurement, availability of resources and machine capacities, and availability of process hours and labor hours. This poor scheduling may lead to severe adverse consequences and might not complete the order fulfillment on time and which badly impacts the company’s prestige.
There are some major drawbacks of poor scheduling is mentioned below,
- Scheduler is unaware of the production capacities
- Scheduler is unaware of the product cycle time
- Scheduling is done without considering the lead time for the procurement of materials
- Wastage due to poor scheduling, as there is underutilization of labor hours and process hours.
- There is a chance of mismanagement and machine wear and tear due to high schedules on specific machines i.e. overcapacity on the ZP27 compression machine.
- There is no performance management system in the supply chain operation.
- Manual calculations may lead to errors
- BOMs have having wrong details, so the material will not be arranged as per the requirement
- There is no KPI to keep an eye on production activity, i.e., not monitoring planned vs achieved production
- The plan is not as per the forecast, or there might be an error in the forecast, leading to over-inventory or shortage of stock in the market
- There is no safety stock for raw material or packaging material
- Delay in imported raw materials due to clearance or late shipments. Scheduler must have flexibility in the production plan
- Ordering extra inventory leads to space constraints in warehouses
- Scheduling does not cover the downtime and preventive maintenance scheduling in the production plan
Figure 5: Pitfalls in scheduling
Concept of Industry 4.0
Industry 4.0 provides automation in supply chain operations, starting from forecasting to the final dispatch. Some of the benefits of Industry 4.0 are listed below,
- Auto calculation of forecast from sales data
- Auto-ordering of raw materials from the authorized suppliers
- Keep inventory of materials under control
- Provides scheduling as per the machine capacity.
- Keeps safety stock of raw and FG inventories.
- Keeps data recordings
Certain software programs are capable of doing MRP, i.e., SAP, Oracle-based software, BPCIS (IBM Software utilized by GSK in earlier days)
Figure 6: Concept of Industry 4.0
Conclusion
In today’s challenging world, supply chain operations have extreme importance because material arrangement has become a challenging task after COVID for many industries. Delay in shipments is common nowadays due to weather conditions, law and order situations of the world and Russia and Russia-Ukraine war, petroleum prices, the Indo-Pak war, and the tariff rate issue.
The world is changing direction, so the decision-making in scheduling is important while planning for the ordering of materials, planning of low-cost products, planning of high-cost products, planning of highly demanded products, and planning of low-demand products. A good planner always keeps an eye on the procurement and sourcing of material from the nearest spots, as he will consider low transportation costs, low cost, and urgent deliveries. As this article also defines the adverse effects of bad scheduling so to avoid any adverse event, a good ERP system must be in place that proposes a good monthly schedule. There must be a remediation plan and flexibility in scheduling to avoid unfortunate conditions.
You can also read about:
Preformulation Study Part(2): Solubility, Stability & Key Parameters in Drug Development