Introduction
In the first part, we reviewed the main classes of impurities, zeroed in on process-related ones, charted their origins and how they affect the API, sketched ways to control them, and noted the regulators and standards overseeing these impurities.
Now, in part two, we turn to degradation products.
Degradation Products
Degradation products form as time passes and either the API or its excipients break down, usually for chemical or physical reasons. Heat, light, damp air, and even oxygen can push these changes along. Keeping the drug stable means watching how fast and how far that breakdown goes
Types of Degradation Product Impurities
Chemical Degradation
Hydrolysis
Hydrolysis is a frequent culprit; water molecules sneak in and sever the bonds that hold the drug together. When that happens, the medicine may lose power or make unsafe by-products. Many esters and amides will quickly in humid air because of this reaction.
Oxidation
Oxidation strips electrons from a molecule, often when it meets oxygen or another strong oxidizer. The result can be new, reactive oxygen species and altered compounds that patients never signed up for. This reaction produces reactive oxygen species (ROS), which can damage either the active ingredient or the inactive materials. Drugs with sulfur atoms, thiazide diuretics, and oils with double bonds are especially prone to this type of oxidation.
Photodegradation
Some medicines break down when exposed to UV light or other radiation, especially if they contain aromatic rings or long conjugated systems. Such exposure alters the molecule’s structure, reducing its effectiveness. Light-resistant packaging can greatly slow this process
Thermal Degradation
Because heat speeds decay, stability tests are done at 30 °C and 40 °C to mimic real storage. Elevated temperatures break chemical bonds, forming unwanted by-products. Products also face high heat during manufacturing and transport, adding to the risk.
Physical Degradation
Polymorphic Changes
A polymorph refers to the existence of a drug in various crystalline forms. Different polymorphs can exhibit variations in solubility and stability. The drug is highly susceptible to transitioning from one polymorph to another during storage, which may impact its performance. For instance, under certain storage conditions, a more soluble polymorph may convert into a less soluble form, thereby affecting it’s bioavailability
Amorphization
refers to how a drug may switch its characteristics over time from being a crystalline structure to non-crystalline (amorphous). This switch can change, among other physical properties, solubility and the extent to which a drug undergoes reconstructive changes due to the specific drug molecule.
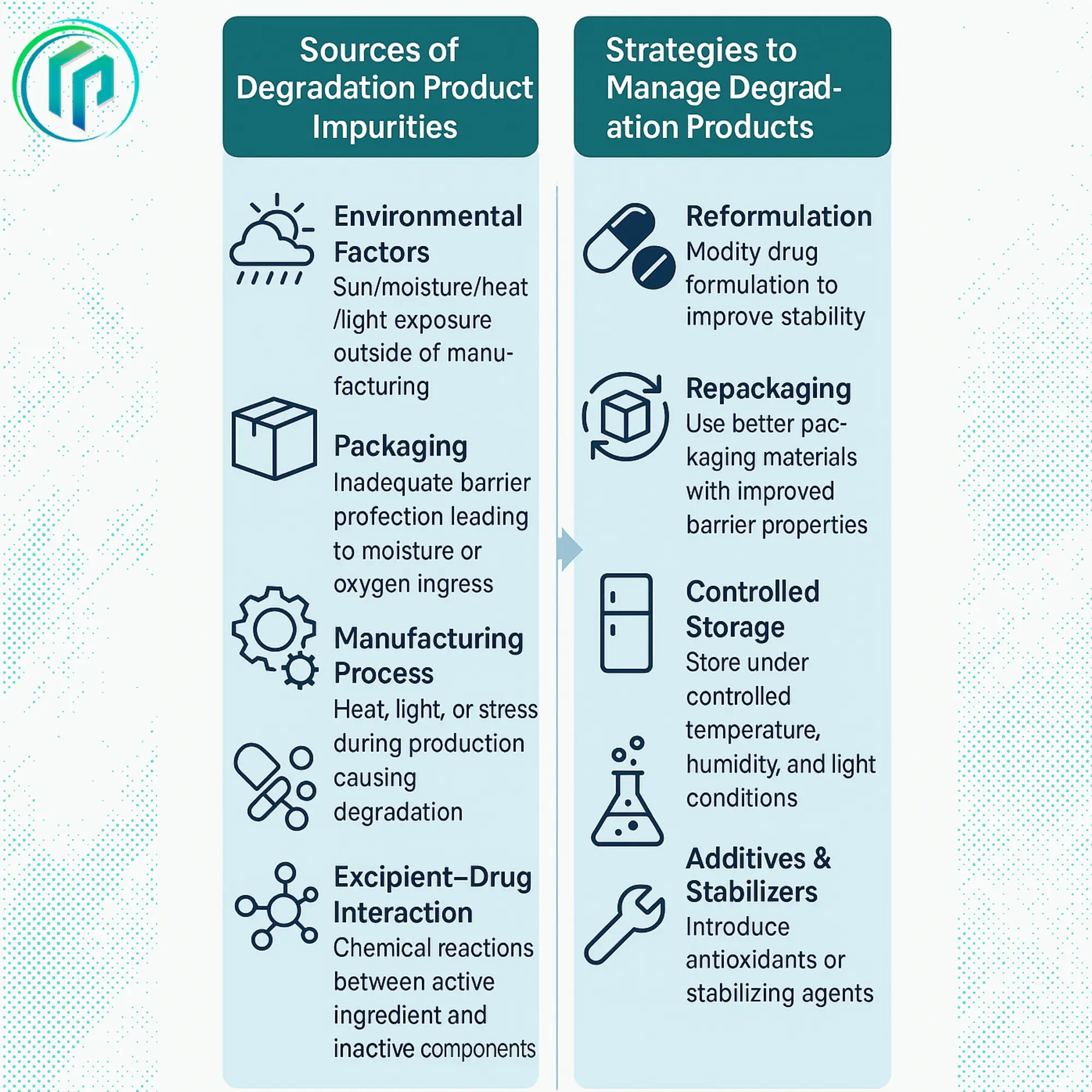
Sources of Degradation Product Impurities
Environmental Factors outside of the manufacturer
The temperature, light, humidity, and oxygen levels that a drug is subjected to while manufactured, transported, or just stored can be contributors.
Drug interactions with excipients
Inactive excipients can increase the degradation of the active pharmaceutical ingredient (API).
The packaging
If the active pharmaceutical ingredient (API) is not packaged properly, it could be sensitive to light or moisture may accelerate the degradation.
The manufacturing process
The manufacturing process itself can create degradation products if there are issues with formulation or handling during production.
Detection of Degradation Products
Various testing methods are employed to ensure stability, efficacy, and quality of drug products.
Stability Studies
These studies are known as accelerated and long-term and are designed to emulate storage conditions such as light, heat, and humidity. They help reveal degradation pathways and different possible degradation products.
Chromatography
Various techniques are used to detect, separate, and quantify degradation products. HPLC (High-Performance Liquid Chromatography) and GC (Gas Chromatography) are the most common methods. Both techniques can identify very small amounts of degradation products, while Gas Chromatography is used only if there are known to be volatile degradation products
Mass Spectrometry (MS)
It identifies products of degradation at the molecular level. It can be a useful tool because it gives precise results for molecular weight and degradation product structure.
Fourier Transform Infrared (FTIR) Spectroscopy
It is used to detect changes in functional groups as a result of degradation. FTIR provides structural information on changes in molecules for the inference of chemical bonds.
Infrared Spectroscopy (IR) and Nuclear Magnetic Resonance (NMR)
It is used to confirm the structures of degradation products.
Thin Layer Chromatography (TLC)
used as a detection method for degradation products, and is a simple and rapid method.
Microscopic and Visual Inspection
Observing the physical appearance of the dosage form (color, shape, etc.) may indicate degradation.
Ways to Manage Degradation Product Impurities

Reformulation of the dosage form
Improving stability involves reformulating the active pharmaceutical ingredient (API), which can be done by altering pH in a sterile dosage form, using certain limited stabilizers.
Repackaging
Using opaque or airtight bottles to protect APIs from light, water, and oxygen.
Controlled Storage
Storing the drug under controlled conditions concerning temperature, humidity, and light
Using Additives and Stabilizers
Using chelators such as EDTA, antioxidants, and stabilizers to protect APIs from degradation.
Improving the Processes
Reducing exposure to conditions that could cause degradation.
Ways to Control Degradation Product Impurities
Quality Control (QC)
Routine analyses of APIs and drug products are important and include determining any significant change in impurity levels within the batches.
Stability Testing
Long-term, intermediate, and accelerated stability studies help monitor the formation of degradation products. It is important to set limits for acceptable impurities for degradation products that are based on toxicology risk assessment
Optimizing the process
The manufacturing process can be improved by limiting temperature or humidity access to reduce the exposure of active pharmaceutical ingredients (APIs) to conditions that may lead to degradation.
Formulation Changes
Changing the formulation by using different excipients or changing the concentration of the active ingredient (API) to minimize degradation.
Effect of Degradants on Active Pharmaceutical Ingredients (APIs)
Safety Concerns
Degradants have potential safety and toxicity, or mutagenicity risks.
Efficacy Concerns
Degradants may lower the active pharmaceutical ingredient (API) potency, potentially causing a therapeutic failure of the drug.
Patient Health Risk
Degradants can also harm the patient if not carefully controlled, especially for the treatment of chronic diseases.
Regulatory Approval
If a regulatory submission or post-marketing surveillance detects a high level of degradants in a drug product, the drug is almost guaranteed to be refused approval based on scientific data alone.
Shelf Life and Stability
Degradants are closely related to shelf life since degradation impacts the longevity of the shelf life.
Regulatory Guidelines and Specifications
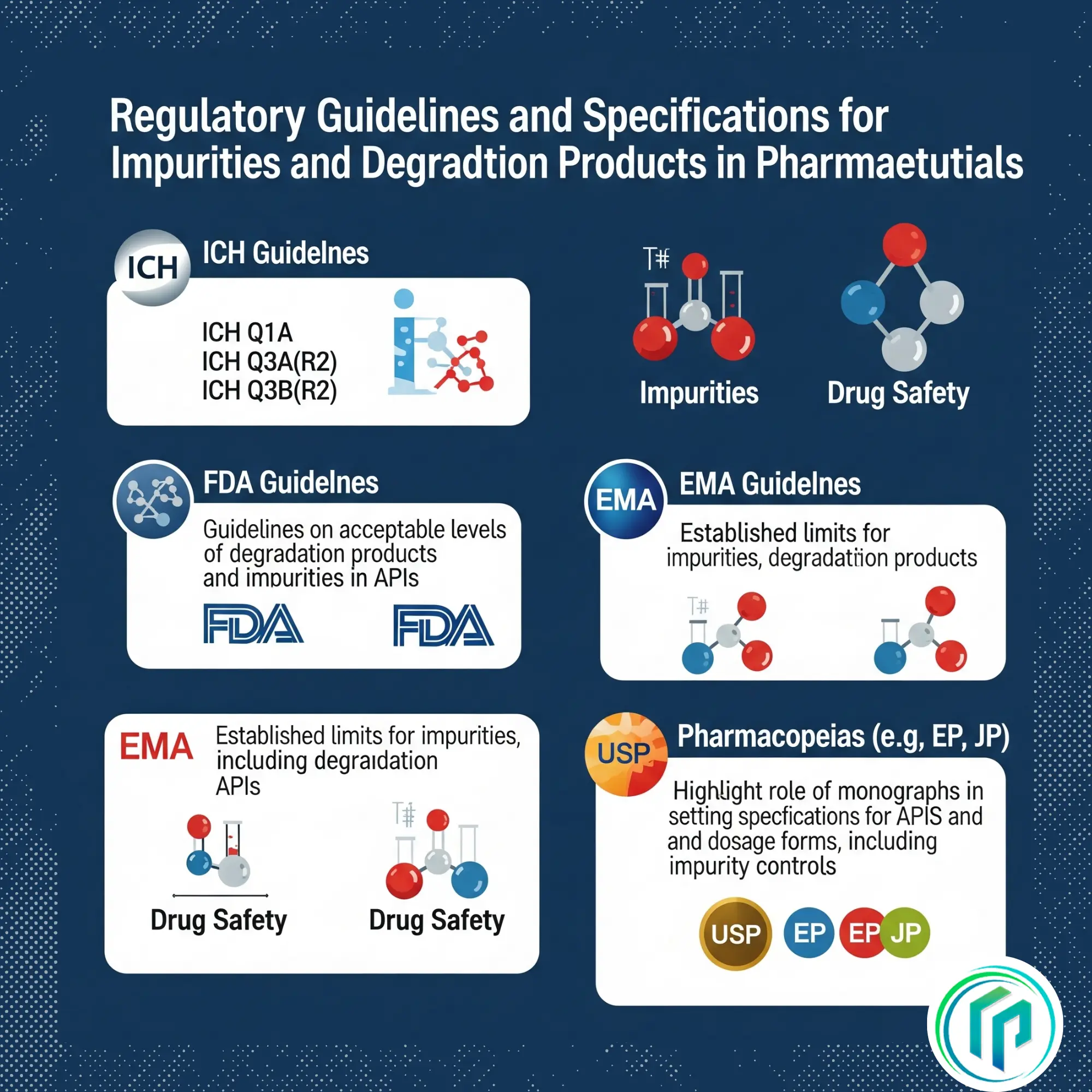
ICH Guidelines (International Council for Harmonization)
– ICH Q1A: Stability testing of pharmaceuticals.
-ICH Q3A(R2): Specifications for organic impurities in drug substances.
– ICH Q3B(R2): Impurity specifications in drug products.
–There are FDA guidelines on acceptable levels of degradation products and impurities in active pharmaceutical ingredients (APIs).
-The European Medicines Agency (EMA) has established limits of impurities (including degradation products)
-The United States Pharmacopeia (USP) and other pharmacopeias have monographs that cover specific active pharmaceutical ingredients (APIs) and dosage forms, and they include specifications of impurities, including degradation products.
Conclusion
Degradant impurities are tremendously troubling to the pharmaceutical industry, especially since they can directly impact the safety, efficacy, and quality of drug products. It is important to be familiar with the types, potential sources, their detection, and regulations surrounding degradation products to deal with them effectively. Following good manufacturing practices, good formulation practices, proper storage, and good testing practices will reduce the impact of degradation products that negatively affect active pharmaceutical ingredients (APIs) and pharmaceutical quality of the final drug product.
References:
Iqbal, Z., Khan, A., & Iqbal, M. S. (2006). Stability-indicating HPLC methods for drug analysis. Journal of Chromatographic Science, 44(3), 93–100.
Blessy, M., Patel, R. D., Prajapati, P. N., & Agrawal, Y. K. (2014). Development of forced degradation and stability indicating studies of drugs—A review. Journal of Pharmaceutical Analysis, 4(3), 159–165. https://doi.org/10.1016/j.jpha.2013.09.003
Pharmaceutical Stress testing: Prediction Drug Degradation
Huynh-Ba, K. (2008). Handbook of stability testing in pharmaceutical development: Regulations, methodologies, and best practices. Springer. https://doi.org/10.1007/978-0-387-85658-2


