What is Knowledge Management (KM)?
In today’s rapidly evolving and complex pharmaceutical field, knowledge is more than information; it’s a strategic asset that drives innovation, ensures regulatory compliance, enhances operational efficiency, supports continuous improvement, and ensures corporate sustainability. Successful pharmaceutical organizations rely on both tacit (experience based) and explicit (documented) knowledge to make informed decisions. This body of knowledge includes R&D know-how, standard operating procedures, production data, stability studies, common technical dossiers, market research studies, and post-marketing feedback.
Knowledge Management is the systematic process of identifying, capturing, organizing, sharing, and effectively using knowledge to achieve organizational objectives. It integrates people, processes, and technology to ensure that knowledge flows efficiently across departments and locations. In pharmaceutical organizations, effective KM enables better decision-making, reduces duplication of effort, accelerates innovation, enhances regulatory compliance, and ultimately contributes to improved patient outcomes and corporate sustainability.
Why is Knowledge Management important?
Pharmaceutical organizations operate in a complex, knowledge-intensive environment. Their success depends heavily on the ability to generate, retain, and utilize knowledge. The strategic importance of KM can be consolidated in the points below.
1. Enhance Innovation:
The journey from discovering a drug molecule or a new formula to launching in the market consumes a lot of time may be years, and costs millions of pounds. KM supports innovation by:
- Facilitate collaboration among researchers.
- Preserve organizational memory.
- Prevent knowledge silos.
- Support data-driven decision-making by providing access to prior experiments and research.
2. Regulatory Compliance:
Pharmaceutical organizations are among the most heavily regulated industries. Agencies like the FDA, ISO, and EU demand meticulous documentation, traceability, and accountability. A robust KM system ensures:
- Proper documentation of Good Manufacturing Practices (GMP).
- Availability of records for audits and inspections.
- Accurate and timely submissions to regulatory bodies.
- Systematic handling of deviations, CAPAs, and quality events.
3. Improve Operational Efficiency:
KM enables organizations to:
- Eliminate redundant efforts by reusing existing knowledge.
- Reduce training time by providing easily accessible knowledge resources.
- Streamline communication and reduce the learning curve for new employees.
4. Support Risk Management:
With complex global supply chains, intensive compliance requirements, and fast-changing market dynamics, risk is a part of daily processes. KM helps in:
- Identifying and sharing best practices and lessons learned.
- Ensuring continuity during employee turnover or crisis events.
- Minimizing human error.
5. Sustaining Competitive Advantage:
In a crowded and competitive market, companies that can best leverage their intellectual capital outperform others. KM contributes by:
- Speeding up time-to-market.
- Improving product quality and reliability.
- Enhancing customer satisfaction through consistent performance.
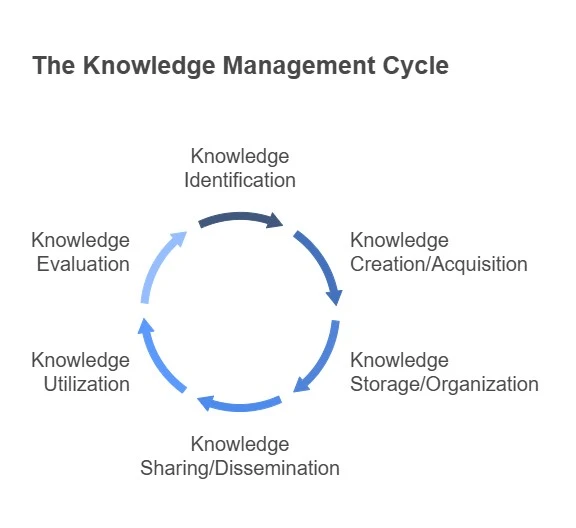
The Process of Knowledge Management
KM is not a one-time project but a continuous process that includes the following stages:
1. Knowledge identification:
The first step is to determine the type of knowledge that exists within the organization and what is needed. Knowledge identification involves mapping intellectual assets, recognizing critical knowledge domains, and determining gaps where knowledge needs to be developed or acquired. This step lays the foundation for all the next activities by helping organizations focus their efforts on high-impact knowledge areas.
2. Knowledge creation and acquisition:
Once knowledge needs are identified, the organization must generate or acquire the needed knowledge. Internally, pharmaceutical companies create knowledge through R&D activities, quality investigations, technology transfers, and continuous improvement initiatives. Externally, they may acquire knowledge through academic partnerships, mergers and acquisitions, scientific publications, or regulatory intelligence. This step is critical in a fast-moving industry where scientific advancements and regulatory updates occur constantly. Facilitating collaborative environments, encouraging cross-functional learning, and maintaining relationships with key external stakeholders are essential to sustaining a healthy pipeline of new and relevant knowledge.
3. Knowledge storage and organization:
Effective KM requires that knowledge be captured and stored in a structured, accessible, and secure manner. This involves using digital repositories such as document management systems, laboratory information management systems (LIMS), and electronic quality management systems. These tools help store a large amount of information. Organizing this knowledge using taxonomies and version control ensures that it can be retrieved efficiently when needed. And due to the industry’s regulatory requirements, storage systems must support data integrity, audit trails, and be compliant with standards like FDA 21 CFR Part 11.
4. Knowledge sharing and dissemination:
Storing knowledge is not enough; it must be actively shared and disseminated to generate value. This step involves making the right knowledge available to the right people at the right time. This involves internal collaboration platforms, interactive dashboards, and knowledge portals.
5. Knowledge utilization:
Utilization is where knowledge is translated into business value. It involves applying the stored and shared knowledge to solve problems, make informed decisions, and drive innovation. Knowledge must be integrated into workflows, decision-making systems, and operational practices to become actionable. A well-utilized KM system leads to fewer errors, reduced rework, and faster time-to-market.
6. Knowledge evaluation:
Ongoing measurement and evaluation are crucial to determine the value of knowledge and the effectiveness of KM initiatives. This involves:
- KPIs like knowledge reuse rate and audit findings.
- Feedback mechanisms.
- Continuous improvement loops.
Knowledge Management Frameworks
Several frameworks guide the implementation of KM in organizations.
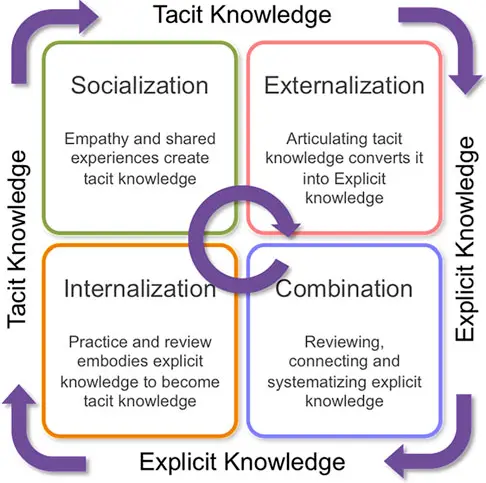
1. SECI Model (Nonaka & Takeuchi):
The SECI model, developed by Ikujiro Nonaka and Hirotaka Takeuchi, is one of the most influential knowledge management frameworks. SECI stands for Socialization, Externalization, Combination, and Internalization. In pharmaceutical organizations, socialization happens when employees informally share their experience (tacit to tacit); externalization is when tacit insights are documented into standard procedures or reports (tacit to explicit); combination involves integrating regulatory updates with internal quality systems (explicit to explicit); and internalization takes place when employees learn from documented procedures or manuals (explicit to tacit). This model emphasizes the importance of converting tacit knowledge, such as intuition, into explicit knowledge that can be institutionalized, reused, and scaled across the organization.
2. The KM Capability Assessment (KMCA) Model:
The KMCA model is a diagnostic framework that evaluates an organization’s readiness and maturity in managing knowledge. It breaks down knowledge management into blocks such as leadership, culture, process, technology, and measurement. Pharmaceutical organizations can use this model to assess the effectiveness of their knowledge management practices in areas like technology transfer, deviation management, or R&D collaboration. A company might score well in technology but poorly in culture if employees are resistant to share failures. By identifying these gaps, the KMCA model helps in setting realistic goals and developing targeted improvement plans and thus driving continuous KM maturity.
3. APQC KM Framework:
Developed by the American Productivity and Quality Center (APQC), this framework organizes organizational processes into a structured hierarchy. It identifies core activities such as developing knowledge strategies, content management, facilitating collaboration, and impact measurement. Pharmaceutical companies often rely on the APQC framework to standardize their KM processes across departments, especially in global operations. The framework also supports benchmarking, making it easier to compare performance against industry peers.
4. ISO 30401:2018:
Developed by the International Organization for Standardization (ISO), this standard provides a comprehensive framework for establishing, implementing, maintaining, reviewing, and improving knowledge management within organizations. Unlike prescriptive guidelines, ISO 30401 adopts a principle-based approach, ensuring flexibility while setting clear expectations for performance. It emphasizes aligning KM efforts with organizational objectives, enabling the creation of value through effective knowledge utilization. The standard covers critical elements such as leadership commitment, stakeholder engagement, culture, processes, and evaluation. All are designed to ensure that knowledge is treated as a core organizational asset. Aligning KM systems with ISO standards strengthens credibility with partners, auditors, and regulatory bodies, asserts the organization’s commitment to operational excellence and continuous improvement.
5. The Cynefin Framework:
Developed by Dave Snowden, the Cynefin Framework classifies issues into five domains: simple (obvious), complicated, complex, chaotic, and disorder. For knowledge management in pharmaceuticals, this model helps determine what kind of knowledge is needed for different situations. The Cynefin framework enables the organization to tailor knowledge strategies based on context, ensuring the right approach for different operational divisions.
Read more: The Cynefin Framework; A Leader’s Framework for Decision Making
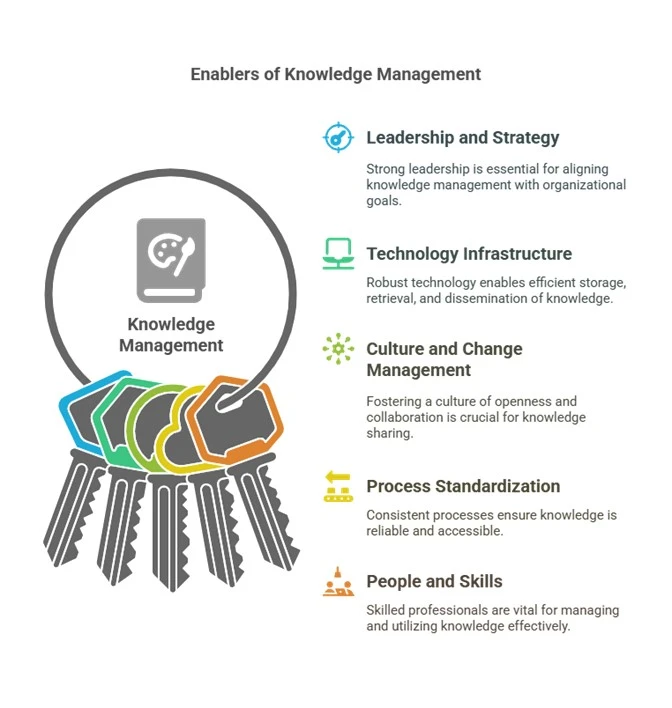
Enablers of Knowledge Management
1. Leadership and Strategy:
Leadership plays a pivotal role in embedding KM into the pharmaceutical organization. Without strong executive sponsorship and strategic alignment, KM initiatives fail. When knowledge management is positioned as a strategic priority, it sends a powerful message across all levels of the organization, encouraging participation and accountability. In high-stakes environments like pharmaceutical R&D or quality control, leadership commitment can be the catalyst that transforms isolated knowledge-sharing efforts into a unified, enterprise-wide capability.
2. Technology Infrastructure:
Technology is the backbone of modern KM systems, enabling the storage, retrieval, and dissemination of vast volumes of data and documents. In pharmaceutical organizations, the technological infrastructure must be robust, scalable, and compliant with industry regulations. Tools such as enterprise resource management systems, document repositories, electronic lab notebooks (ELNs), and laboratory information management systems (LIMS) facilitate structured knowledge capture and retrieval. Emerging technologies such as artificial intelligence enhances knowledge management by offering predictive insights and contextual relevance. However, technology alone is not sufficient; it must be user-friendly, interoperable, and integrated seamlessly into daily workflows to be effective.
3. Culture and Change Management:
A knowledge-sharing culture is the most intangible but arguably the most critical enabler of knowledge management success. In pharmaceutical organizations where knowledge means corporate sustainability, fostering a culture that encourages openness, collaboration, and continuous learning is essential. Employees need to feel safe and rewarded for sharing insights, reporting failures, and mentoring others. Change management becomes crucial here, as shifting mindsets from “knowledge is power” to “sharing is power” often meets resistance. This requires sustained communication, leadership role-modeling, training, and change champions who reinforce desired behaviors.
4. Process Standardization:
Effective KM depends on consistent, repeatable processes for knowledge capture, validation, storage, and dissemination. Standard operating procedures and workflows provide the structure necessary to ensure that knowledge is not only recorded but is also trusted and accessible. Well-defined KM processes reduce variability, prevent knowledge loss, and help embed learning into the organizational DNA.
5. People and Skills:
People are the center of any KM system, both as contributors and users of knowledge. Having the right talent, including knowledge managers, data scientists, IT support, and subject matter experts, is essential. These individuals facilitate the flow of knowledge, ensure quality, and mentor teams in KM practices. In the pharmaceutical industry, where scientific precision and regulatory compliance are critical, skilled professionals ensure that knowledge is both relevant and reliable. Empowering people to become active participants in KM creates a cycle of learning and improvement.
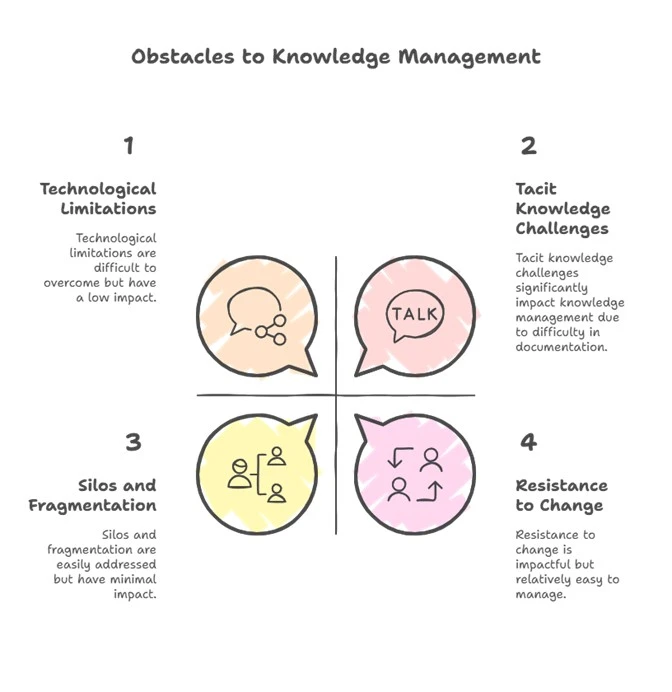
Obstacles to Knowledge Management
Despite its value, KM faces several barriers in pharmaceutical organizations:
1. Silos and Fragmentation:
Departments often operate in isolation, leading to duplicated efforts and inconsistent practices.
2. Resistance to Change:
Employees may be reluctant to share knowledge due to fear of redundancy or loss of control.
3. Tacit Knowledge Challenges:
Much critical knowledge is experience-based and hard to document.
4. Technological Limitations:
Inadequate systems, lack of interoperability, or poor user experience can hinder knowledge sharing.
5. Overload and Irrelevance:
Too much poorly organized information can overwhelm users, leading to underutilization of KM systems.
How to implement KM in your organization?
1. Assessment and Vision:
- Conduct a knowledge audit.
- Map knowledge assets and gaps.
- Align the KM activities with business goals.
2. Strategy and Planning:
- Define objectives and scope.
- Prioritize the knowledge.
- Form a KM team and governance structure.
3. Design and Implementation:
- Choose appropriate frameworks and tools.
- Establish taxonomies.
- Design processes for knowledge creation, storage, and sharing.
4. Change Management and Training:
- Promote a culture of knowledge sharing.
- Provide incentives and recognition.
- Offer training in KM.
5. Monitoring and Improvement:
- Define KPIs.
- Regularly evaluate process effectiveness.
- Iterate and scale up successful practices.
Final Tip: Finding the Balance between Innovation and Organizational structure
A balance between innovation and organizational structure should be the desired outcome of a good KM strategy. The fluid intellectual domain consists of individuals with ideas originating and growing from intuition and experience. Innovation thrives in environments that encourage experimentation, cross-functional collaboration, and the freedom to explore unconventional solutions. In contrast, the organization strives to structure work and to control processes and measure outcomes. If the organization is too fluid, there will be no solid connection of innovative work to business goals, and the organization will lack proper accountability. On the other hand, if the balance shifts too much in favor of institutionalization, the organization will be too formal, leading to innovation and communication barriers.
A successful balance requires clear boundaries and seamless integration. Innovation must not be siloed or treated as a separate function; instead, it should be embedded within the organizational fabric, supported by knowledge-sharing systems, leadership, and an adaptive culture. At the same time, the organizational structure should ensure that innovative ideas are evaluated, scaled, and integrated efficiently into core operations without compromising compliance, safety, or quality. When structure and innovation are harmonized, companies can accelerate drug development, improve patient outcomes, and maintain a sustainable competitive edge.
Conclusion
In the knowledge-driven world of pharmaceuticals, effective KM is a necessity. It supports every aspect of the business, from innovation and compliance to efficiency and competitiveness. By embracing a structured, strategic approach to knowledge management with proven frameworks and a supportive organizational culture, pharmaceutical organizations can unlock their full intellectual potential and create sustained value for patients and stakeholders.