Introduction
Mass spectrometry (MS) is a cornerstone in the world of analytical chemistry, providing a powerful tool to reveal the secrets of chemical compositions, molecular structures, and concentrations. At the heart of this technique lies ionization, which is the process that transforms neutral molecules into charged particles, making them detectable and ready for in-depth analysis. The way this transformation occurs is crucial, as it influences the sensitivity, precision, and versatility of the entire method. In this article, we will take a deep dive into the fascinating world of ionization techniques, exploring their mechanisms and revealing how each one shapes the landscape of mass spectrometry.
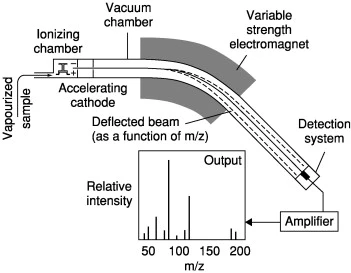
Figure 1: Mass spectrometry diagram
What is Ionization in Mass Spectrometry, and why is it essential?
Ionization refers to the process of turning neutral molecules or atoms into ion, charged species that can be manipulated by electric and magnetic fields within the mass spectrometer. The primary function of ionization in mass spectrometry is to facilitate the measurement of the molecular weight, structure, and other properties of compounds. Ionization is essential because mass spectrometers can only detect ions, not neutral molecules.
Classification of ionization
Ionization methods in mass spectrometry are classified based on how ions are generated, the type of ions produced, and the degree of fragmentation. The choice of ionization method significantly impacts the sensitivity, resolution, and applicability of the mass spectrometry analysis. These methods can be divided into two categories: hard ionization and soft ionization techniques, which differ in their degree of fragmentation and their suitability for different types of samples.
➤ Hard Ionization
Hard ionization uses high ionization energy, which causes the fragmentation of the analyte molecules, producing not only the molecular ion but also other fragment ions. These methods are often used for volatile and stable compounds such as gases, small organic molecules, and solvents, where detailed fragmentation is valuable for structural determination. The most commonly used hard ionization technique is Electron Ionization
Electron Ionization (EI)
Also known as electron impact ionization, it is one of the oldest and most widely used ionization techniques
Mechanism
In EI, a sample is introduced into a high-energy electron beam within an ionization chamber, where electrons are generated by a heated filament (cathode) that emits electrons typically having an energy of 70 eV when heated. The sample molecules are bombarded with these electrons. This interaction leads to the ejection of an electron from the sample molecule, generating a radical cation (M⁺) known as “the molecular ion or parent ion (M⁺)”. The general equation for this process is:
M + e− → M+. + 2e−
Where:
- M is the molecule (neutral) being ionized.
- e⁻ represents an electron from the electron beam.
- M⁺. is the positively charged ion formed (radical cation).
- The second e⁻ is the electron ejected during ionization.
and sometimes secondary ions, “fragment ions”.
Radical cations can fragment by:
a) elimination of a neutral radical M +. —> A + + B. or by
b) elimination of an even-electron molecule M +. —> C +. + D
The ionized molecules are then accelerated into the mass analyzer for detection
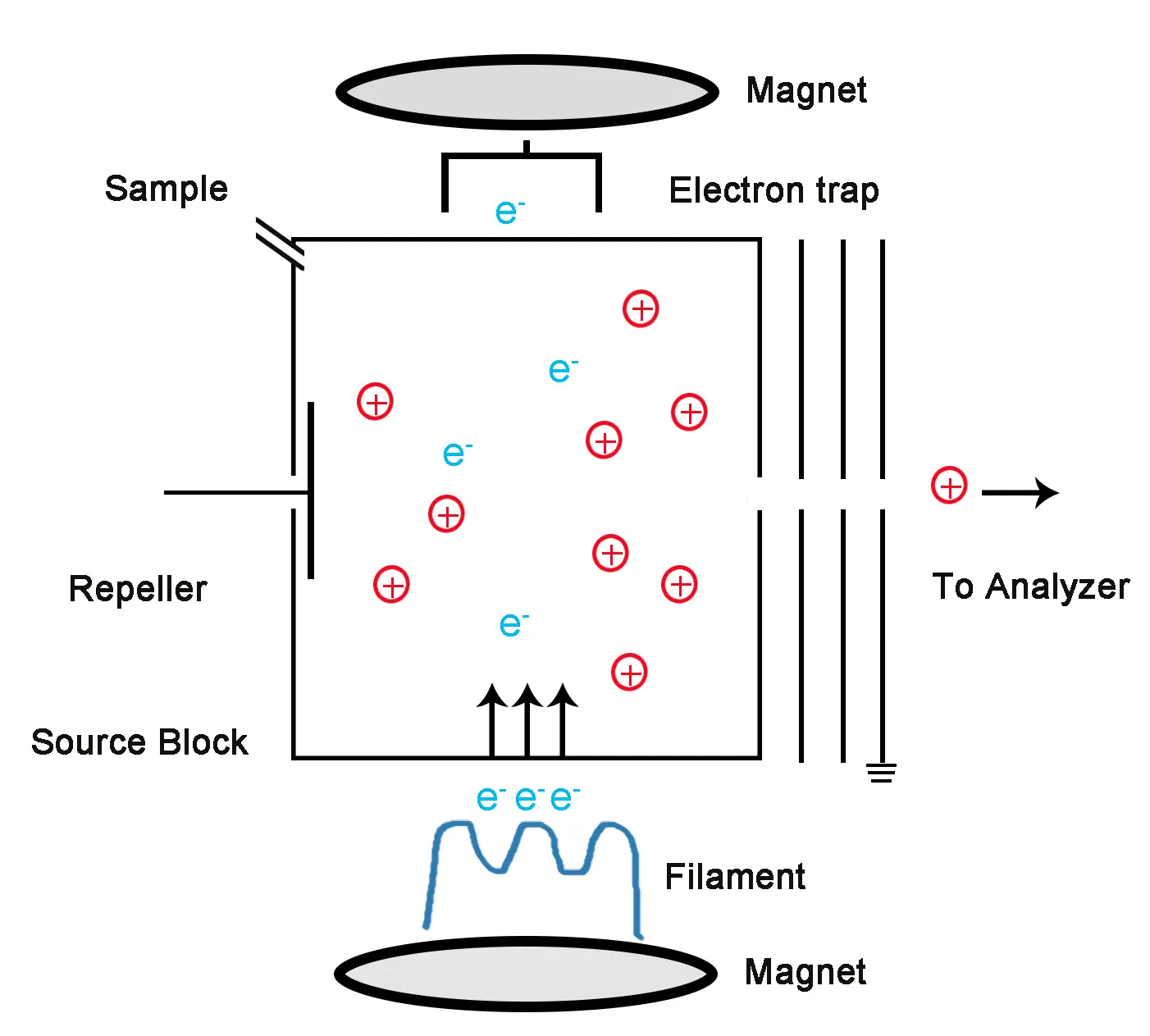
Figure 2: Electron ionization Diagram
Application
Commonly used in conjunction with Gas chromatography (GC-MS) for analyzing small organic molecules such as hydrocarbons, alcohols, and aromatic compounds.
| Advantages | Limitations |
| Well-established and widely available | Not suitable for large or thermally labile compounds |
| Provides structural information through fragmentation patterns | It can lead to excessive fragmentation, losing information about molecular weight. |
➤Soft ionization
Soft ionization methods have the advantage of generating ions with minimal fragmentation, leading to the preservation of the molecular integrity of the analyte for precise analysis. These techniques are particularly valuable in the study of complex biomolecules, such as proteins, nucleic acids, and large organic compounds, where maintaining the original molecular structure is crucial
❖Electrospray Ionization (ESI)
It is commonly used in conjunction with liquid chromatography (LC-MS) for complex sample analysis.
Mechanism
The electrospray ionization process begins with the generation of charged droplets from a liquid sample under a strong electric field. The key stages of this process are:
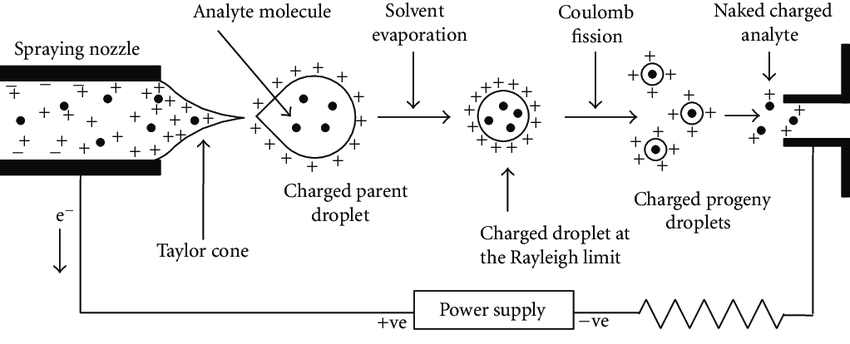
Figure 3: Electrospray Ionization diagram
- Sample Introduction: where The sample is typically dissolved in a solvent that is compatible with both the sample and the mass spectrometer. The solvent is often a mixture of water, organic solvents (e.g., acetonitrile), and acid (e.g., formic acid) to help in ionization.
- Formation of Charged Droplets: A high voltage (typically 3–5 kV) is applied to a metal needle or capillary through which the sample solution is pumped. This voltage causes the solution at the needle tip to break up into a fine spray of charged droplets. The spray is produced due to the electrostatic forces acting on the liquid.
- Desolvation: The charged droplets enter a heated region of the mass spectrometer known as the “desolvation” or “drying” zone, where the solvent begins to evaporate. As the solvent evaporates, the droplets become smaller in size, causing higher charge density.
- Coulombic Explosion and Ion Formation: Eventually, the charged droplets undergo a process known as Coulombic explosion, where the repulsive forces between like charges cause the droplets to break apart even more, ultimately releasing gas-phase ions. These ions are the analytes of interest, typically in the form of singly or multiply charged species, depending on the molecular weight and structure of the analyte.
- Ion Transfer: The ions are then transferred into the mass spectrometer for analysis, where they are subjected to further manipulation, such as mass filtering or fragmentation.
Application
Works well with polar and non-volatile compounds: Ideal for peptides, proteins, and large biomolecules.
| Advantages | Limitations |
| Can produce both positive and negative ions: The polarity of the ions can be controlled based on the analysis | The technique is sensitive to sample contamination (e.g., salts). |
| Soft ionization reduces fragmentation, providing intact molecular ions. | Limited to polar and water- soluble compounds. |
❖Matrix-Assisted Laser Desorption/Ionization (MALDI)
The technique combines laser desorption with matrix-assisted ionization to generate gas-phase ions suitable for mass spectrometric analysis.
Mechanism
- Sample Preparation:
In MALDI, the analyte (the substance to be measured) is first co-crystallized with a matrix material, typically an organic compound that absorbs UV light. The matrix is chosen for its ability to absorb the laser energy and transfer it efficiently to the analyte.
The sample is then applied to a solid metal plate, typically made of stainless steel, and allowed to dry. The dried sample is a thin layer composed of the matrix and analyte molecules.
- Laser Irradiation:
A laser, usually operating in the UV range (e.g., 337 nm from a nitrogen laser), is directed onto the sample. The matrix absorbs the energy from the laser and undergoes rapid heating, causing it to vaporize.
As the matrix evaporates, it transfers energy to the analyte molecules. The absorbed energy leads to the desorption and ionization of the analyte.
The energy is typically transferred via a protonation or deprotonation mechanism, depending on the matrix and the conditions, leading to either positive or negative ions of the analyte.
- Ionization:
The matrix acts as both a light absorber and a facilitator of ion formation. In MALDI, the analyte molecules typically undergo protonation, forming positively charged ions. However, in some cases, negative ions can also be formed depending on the nature of the matrix and analyte.
The energy transfer from the matrix to the analyte may cause the analyte to form ions in its molecular form (e.g., [M+H]+ for positive ions or [M-H]– for negative ions). The process occurs without the need for additional reagent gases, and ionization typically occurs in the gas phase.
- Ion Acceleration:
Once ionized, the analyte ions are accelerated by an electric field within the mass spectrometer. The acceleration separates ions based on their mass-to-charge ratio (m/z).
The time-of-flight (TOF) mass spectrometer is commonly used in conjunction with MALDI. In a TOF spectrometer, ions are accelerated by a voltage and then travel through a flight tube. Lighter ions reach the detector faster than heavier ions, allowing for the determination of the m/z ratio.
- Detection:
The separated ions are then detected using a variety of detectors, such as a time-of-flight detector (TOF) or quadrupole detectors. The intensity of the signal is proportional to the number of ions of a particular m/z ratio, and this data is used to generate a mass spectrum.
The resulting mass spectrum can be analyzed to determine the molecular weight of the analyte, as well as other structural information.
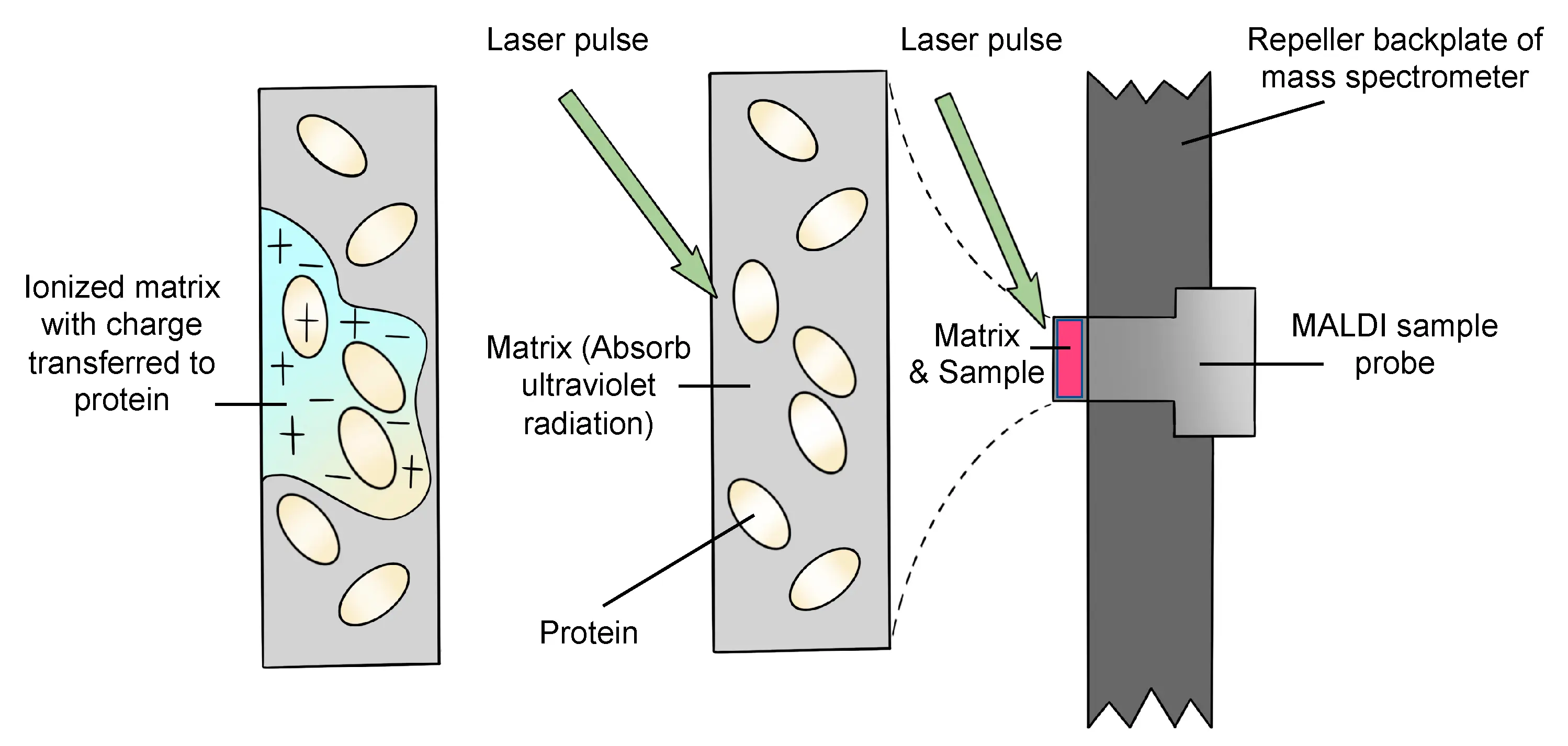
Figure 4: Matrix-Assisted Laser Desorption/Ionization (MALDI) diagram
Application
- Proteomics: For protein identification and characterization.
- Metabolomics: In analyzing metabolic profiles.
- Polymer Analysis: For studying synthetic polymers and their molecular weights.
- For identifying biomolecular markers.
| Advantages | Limitations |
| High Sensitivity: It can detect low-abundance species with high sensitivity, making it suitable for complex biological samples. | Matrix interferences can sometimes affect the analysis. |
| High Sensitivity: it can detect low-abundance species with high sensitivity, making it suitable for complex biological samples. | Limited to solid-state or semi-solid samples. |
❖Atmospheric Pressure Chemical Ionization (APCI)
Atmospheric Pressure Chemical Ionization (APCI) is an ionization technique used in mass spectrometry that operates under atmospheric pressure conditions, making it highly effective for the analysis of thermally labile and non-volatile compounds. APCI is commonly coupled with liquid chromatography (LC) systems
Mechanism
Ion Source Setup:
- The sample enters the APCI source as a fine aerosol from a liquid chromatography (LC) system. A small flow of solvent carries the analyte into the ionization chamber.
- In the ionization chamber, a corona discharge needle is used to apply a high voltage to produce ions from the solvent. The corona discharge creates a plasma, which ionizes the solvent molecules, generating reactive ions (such as protons, electrons, and radicals).
Corona Discharge and Ionization:
- The corona discharge produces a high-density region of charged particles, primarily in the form of positive ions like [H]^+ (protons), which can then interact with the sample molecules.
- The most common ionization process involves the transfer of a proton (H^+) from the corona discharge-generated ions to the analyte molecule, forming a protonated molecular ion ([M+H]^+) for positive ion mode. This is a crucial step in generating ions that can be analyzed in the mass spectrometer.
Gas Phase Reactions:
- After the corona discharge, the ionized molecules interact with neutral solvent molecules (e.g., water or methanol) in the gas phase. These reactions involve the transfer of charge between the solvent ions and the analyte, or even the formation of solvated ions, where the analyte is associated with one or more solvent molecules.
- If the analyte is volatile, it can be ionized directly by the corona discharge. However, in cases where the analyte is less volatile, it may be assisted by vaporization or solvent ionization.
Desolvation:
- The gas-phase ions are passed through a heated region known as the desolvation zone, where the solvent molecules are evaporated and removed from the analyte ions. This ensures that only the analyte ions, free from solvent molecules, enter the mass spectrometer for analysis.
- Desolvation is crucial to ensure that the ions are in their gas-phase form and are ready to be analyzed by the mass spectrometer.
Ion Transfer to Mass Spectrometer:
- The ionized analyte enters the mass spectrometer, where it is accelerated and analyzed based on its mass-to-charge ratio (m/z). The ions are typically analyzed using quadrupole, time-of-flight (TOF), or ion trap mass spectrometers.
- The ions are detected by a mass analyzer, generating a mass spectrum where the peaks represent the different analytes and their respective ionization states.
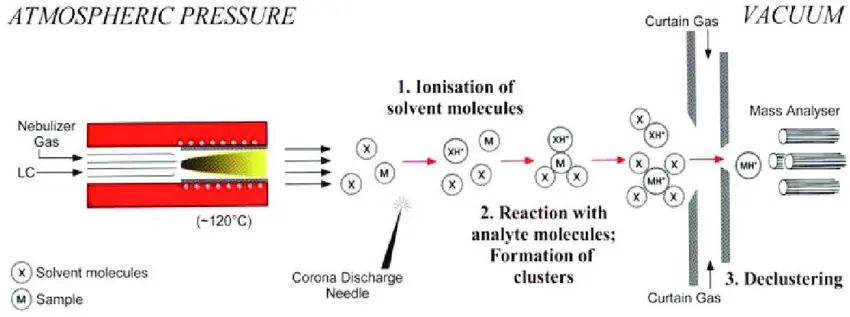
figure 5: Atmospheric Pressure Chemical Ionization (APCI)
Applications
- Analysis of drug compounds, metabolites, and impurities. It is suitable for compounds that may be thermally labile or have low volatility.
- Analysis of pesticides, herbicides, and other environmental contaminants
- Identification and quantification of contaminants, preservatives, and additives in food products.
- Analysis of biomarkers, therapeutic drugs, and metabolites in biological samples.
| Advantages | Limitations |
| High Sensitivity: making it suitable for low-concentration samples, especially when coupled with liquid chromatography (LC). | Limited Ionization Efficiency for Polar Compounds: APCI tends to be less efficient at ionizing highly polar compounds, particularly those that do not easily protonate. |
| It is effective for small to medium-sized molecules that are volatile or semi-volatile, making it widely used in pharmaceutical, environmental, and chemical analysis. | Solvent Dependence: The choice of solvent can influence the efficiency of ionization in APCI. Solvent choice needs to be optimized for the specific analyte being analyzed. |
| APCI is commonly coupled with liquid chromatography (LC-APCI-MS) for separating complex mixtures before ionization, allowing precise quantification and structural identification of compounds. | Matrix Effects: like other ionization techniques, matrix effects (interference from the solvent or other components in the sample) can affect the accuracy of quantification. |
❖Chemical Ionization
Chemical ionization (CI) is an ionization technique in mass spectrometry (MS) that involves the interaction between a sample molecule and a reagent gas, typically resulting in the formation of ions from the sample. Unlike electron impact ionization (EI), which directly ionizes the sample by bombarding it with high-energy electrons, CI provides a softer ionization method, generating ions with lower fragmentation.
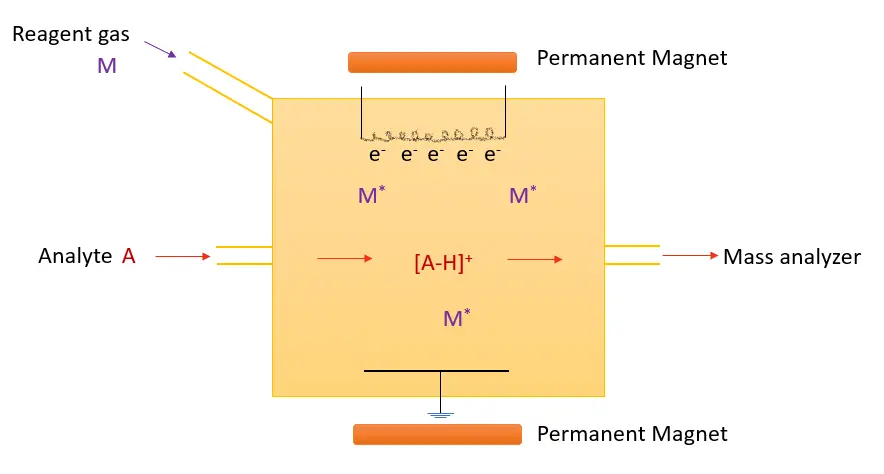
Figure 6: Chemical Ionization Diagram
Mechanism
- Reagent Gas Introduction:
A reagent gas, such as methane (CH₄), ammonia (NH₃), or isobutane (C₄H₁₀), is introduced into the ionization chamber. The reagent gas is typically ionized by electron impact (e.g., by bombarding it with high-energy electrons) to generate reagent ions, such as CH₄⁺ or NH₄⁺.
- Ion-Molecule Reaction:
These reagent ions then interact with the sample molecules through various ion-molecule reactions. These reactions result in the formation of ions from the sample, typically in the form of molecular ions (M⁺) or adduct ions (e.g., [M + H]⁺, [M + CH₄]⁺). The degree of ionization depends on the chemical properties of the analyte and the reagent gas.
- Formation of Product Ions:
The ions formed in the ionization chamber are then accelerated into the mass spectrometer for analysis. The ions can undergo further fragmentation in the ion trap or other ion sources if required.
Types of Chemical Ionization:
- Positive Chemical Ionization (PCI): In PCI, the reagent gas is ionized to form positively charged ions, which interact with the analyte molecule. This method is suitable for positively charged analytes, such as those containing basic functional groups (e.g., amines).
- Negative Chemical Ionization (NCI): In NCI, the reagent gas is ionized to form negatively charged ions, which then interact with the analyte. This mode is useful for compounds that have electron-accepting properties, such as halogenated compounds, organic acids, and nitro compounds.
Application
Identification of Unknown Compounds, specifically small molecules like pharmaceuticals, environmental samples, and food products.
| Advantages | Limitations |
| Higher Sensitivity since it produces fewer fragments and more intact molecular ions, it is particularly useful for detecting compounds with low volatility or compounds that are easily fragmented by EI. | Less informative in terms of fragmentation patterns compared to EI. |
Conclusion
Ionization is a critical step in mass spectrometry, and the choice of ionization method depends on the nature of the sample, the type of analysis, and the level of fragmentation required. Each ionization technique: Electron Impact Ionization (EI), Electrospray Ionization (ESI), Matrix-Assisted Laser Desorption/Ionization (MALDI), Chemical Ionization (CI), and Atmospheric Pressure Ionization (API) offers distinct advantages and limitations. Understanding the different ionization methods is essential for selecting the appropriate technique to achieve the best results for any specific mass spectrometric analysis.
References
- Gross, M. L. (2004). “High Performance Mass Spectrometry: Chemical Applications.” Elsevier Academic Press.
- Smith, R. D., & Kelleher, N. L. (2008). “Advances in Mass Spectrometry: A Review of Mass Spectrometry Techniques.” Biochimica et Biophysica Acta.
- Harris, D. C. (2015). “Quantitative Chemical Analysis.” 9th edition. W. H. Freeman.
- Yost, R. A., & Enke, C. G. (1978). “Chemical Ionization”
- Lioy, P. J., et al. (2010). “Mass spectrometry in the 21st century: Applications to environmental and biomedical studies.” Environmental Health Perspectives, 118(6), 765-776. https://doi.org/10.1289/ehp.0901790
- Bresson, S., et al. (2007). “The Role of Ionization Techniques in Mass Spectrometry.” Journal of Mass Spectrometry, 42(5), 503-512.
- Hill, H. H. (2003). “Ionization Methods in Mass Spectrometry.” Mass Spectrometry Reviews, 22(4), 267-296.
- McLafferty, F. W., & Tureček, F. (1993). Interpretation of Mass Spectra. University Science Books.
- Beynon, J. H., & McLaren, J. (1984). Electron Ionization Mass Spectrometry: Instrumentation and Techniques. Academic Press.
- Frey, J. G. (1994). Introduction to Mass Spectrometry: Instrumentation, Applications, and Techniques. Wiley-Interscience.
- Creative Proteomics. (n.d.). Electron ionization. Creative Proteomics. Retrieved July 11, 2025, from https://www.creative-proteomics.com/support/electron-ionization.htm
- ScienceDirect. (n.d.). Mass spectrometry. ScienceDirect. Retrieved July 11, 2025, from https://www.sciencedirect.com/topics/physics-and-astronomy/mass-spectroscopy
- Han, X., & Emmett, M. R. (2004). “Electrospray ionization: A powerful tool for proteomics.” Analytical Chemistry, 76(4), 885-893. https://doi.org/10.1021/ac0356283
- Cole, R. B. (2000). Electrospray Ionization Mass Spectrometry: Fundamentals, Instrumentation, and Applications. Wiley-Interscience.
- Zhang, Z., et al. (2007). “Applications of electrospray ionization mass spectrometry in pharmaceutical analysis.” Journal of Pharmaceutical and Biomedical Analysis, 44(3), 562-576. https://doi.org/10.1016/j.jpba.2007.02.010
- Banerjee, Shibdas & Mazumdar, Shyamalava. (2012). Electrospray Ionization Mass Spectrometry: A Technique to Access the Information beyond the Molecular Weight of the Analyte. International journal of analytical chemistry. 2012. 282574. 10.1155/2012/282574.
- Cole, R. B., et al. (1997). Electrospray ionization mass spectrometry: A review. Journal of Mass Spectrometry, 32(1), 25–40.
- Fenn, J. B., et al. (1989). Electrospray ionization for mass spectrometry of large biomolecules. Science, 246(4926), 64–71.
- Karas, M., & Hillenkamp, F. (1988). Laser desorption ionization of proteins with molecular masses exceeding 10,000 daltons. Analytical Chemistry, 60(20), 2299–2301.
- Cech, N. B., & Enke, C. G. (2001). Practical implications of some recent developments in ionization techniques for mass spectrometry. Analytical Chemistry, 73(2), 278–287.
- Wu, J., Rong, Z., Xiao, P., & Li, Y. (2022). Imaging Method by Matrix-Assisted Laser Desorption/Ionization Mass Spectrometry (MALDI-MS) for Tissue or Tumor: A Mini Review. Processes, 10(2), 388. https://doi.org/10.3390/pr10020388
- Karas, M., & Krüger, R. (2003). Ionization in Mass Spectrometry. Analytical and Bioanalytical Chemistry, 376(8), 945–950. doi: 10.1007/s00216-003-1741-2.
- Hillenkamp, F., & Karas, M. (2007). Mass Spectrometry of Biopolymers. Analytical Chemistry, 79(1), 36-50. doi: 10.1021/ac0712614.
- Caprioli, R. M., et al. (1999). MALDI-TOF Mass Spectrometry: A Powerful Tool for Biomolecule Identification. Nature Biotechnology, 17(11), 1139-1144. doi: 10.1038/14160.
- Dubey, Naveen. (2020). Metabolomics. 10.5772/intechopen.92423.
- Zhang, Y., & Li, J. (2017). “Principles and Applications of Atmospheric Pressure Chemical Ionization (APCI) in Mass Spectrometry.” Mass Spectrometry Reviews, 36(4), 597-625. doi:10.1002/mas.21468.
- Cech, N. B., & Enke, C. G. (2001). “Atmospheric Pressure Chemical Ionization.” Mass Spectrometry Reviews, 20(6), 503-520. doi:10.1002/mas.10047.
- Zheng, J., et al. (2019). “Recent Advances in Atmospheric Pressure Ionization Techniques for Mass Spectrometry.” Trends in Analytical Chemistry, 118, 52-66. doi:10.1016/j.trac.2019.04.006.
- Gross, M. L. (2011). High Performance Mass Spectrometry: Chemical Applications. John Wiley & Sons.
- Wikipedia contributors. (n.d.). Chemical ionization. Wikipedia. Retrieved July 12, 2025, from https://en.wikipedia.org/wiki/Chemical_ionization