Introduction
Gas Chromatography (GC) is a powerful analytical technique first introduced by Nobel Laureate Archer J.P. Martin and his colleagues in 1952. More than 60 years later, GC is now widely commercialized and essential tool across multiple industries. It enables both qualitative and quantitative analysis with high accuracy. In the pharmaceutical industry, GC is primarily used for residual solvent analysis.
Gas Chromatography (GC) is a technique of separation, identification, and quantification of components in a mixture. It is especially useful for analyzing volatile and partially-volatile compounds that can easily turn into gas.
The basic principle is simple:
Different compounds are carried by the gaseous mobile phase through a column at different speeds due to many differences related to their unique chemical properties. These differences make it easier for a detector to detect them separately, but before going through method development, first we need to discover the GC system
Components of a GC System
After we know the principle, we need to get a quick overview of the fantastic 4 components of a Gas Chromatography System : Sample Injection, Stationary Phase, Carrier Gas and Detectors.
Sample injection
So, how the sample is injected may happen in different ways according to :
- Portion of sample to be injected into :
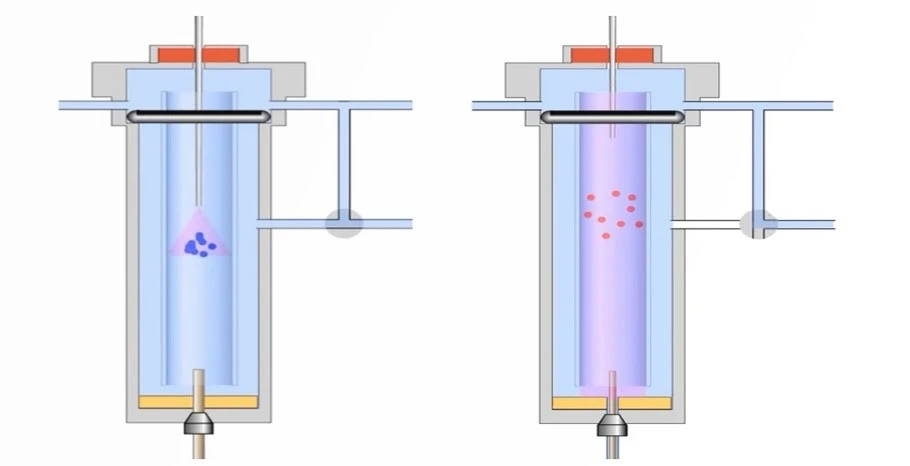
- Split injection: only a portion of the sample is split away from the column by a split vent. It is mainly used when the concentration of the sample is relatively high and high sensitivity is required.
- Split-less injection: the entire sample is vaporized and transferred into the column it is mainly used for trace-level samples or when high sensitivity is required.
- The technique of injection includes :
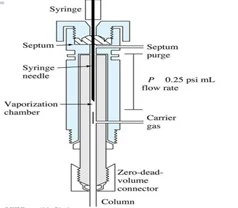
- Direct injection: simply transfer the sample directly into the column without pre-vaporization. This is mainly used for thermally labile or high-boiling-point components to avoid degradation or adsorption onto the injection port or liner during vaporization.
- Programmable Temperature Vaporization (PTV) Injection: PTV injection is used for the controlled vaporization and injection of large sample volumes. The sample is first trapped in a cooled liner and then rapidly heated to vaporize the analytes, providing enhanced sensitivity for trace analysis.
Stationary phase
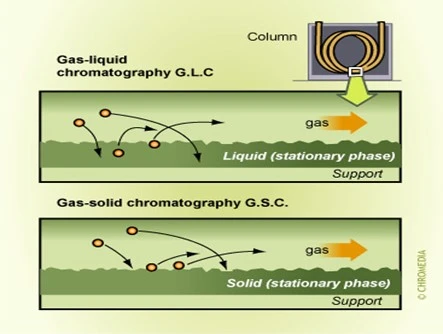
Gas-solid columns (GSC): the column is packed with solid material, Common solid stationary phases include porous polymers, silica gel, molecular sieves, and activated charcoal. molecules interact with the solid surface through adsorption, and separation is achieved based on differences in their affinity for the stationary phase.
Gas-liquid columns (GLC): the stationary phase is a liquid film that is coated on a solid support material, “the column packing”. The liquid phase is typically a nonvolatile, high-boiling-point liquid that is chemically bonded to the surface of the solid support. Common liquid stationary phases such as polyethylene glycol (PEG), polydimethylsiloxane (PDMS), and cyanopropyl phenyl. Separation is achieved through a combination of adsorption and partitioning of the sample molecules between the liquid film and the mobile phase.
Carrier Gas
It represents the mobile phase, carrying the sample through the column for separation and detection. So, how do we choose the carrier gas?
The choice of carrier gas depends on various factors, such as the nature of the analytes, the type of column, and the detector used. And here are some commonly used carrier gases :
Helium (He):
Helium is the most common used carrier gas in GC, as inert gas that avoid interaction with sample analyzed , low-viscous gas, suitable for most of detectors so it offers excellent separation.
Hydrogen (H₂):
Hydrogen is another popular choice as a carrier gas. Its low viscosity and high diffusion rate enable for faster analysis compared to helium. It’s especially effective for analyzing small molecules like hydrocarbons and volatile organic compounds. However, since hydrogen is flammable, using it requires extra safety measures.
Nitrogen (N₂):
Nitrogen is an inert and cost-effective carrier gas often used in routine analyses, especially for stable or non-reactive compounds. While it’s not as efficient as helium or hydrogen in terms of speed and resolution, it’s compatible with many detectors, though it’s not ideal for detectors like flame ionization detectors (FID), which perform better with more ionizable gases.
Argon (Ar):
Argon has higher viscosity and density compared to helium can result in longer retention times.
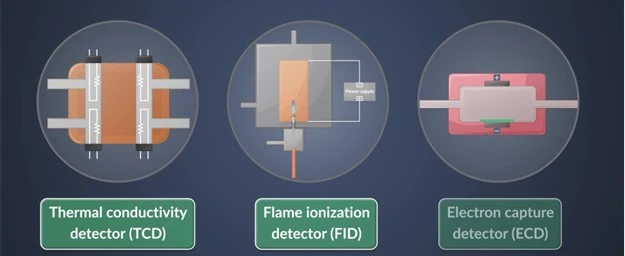
Detectors
Now we need to detect the components that eluted from the column. Let’s discover the most used detectors :
- Flame Ionization Detector (FID): one of the most common used detectors and universal detector for organic compounds . It ionizes organic molecules in a hydrogen/air flame and measuring the resulting ion current. It is highly sensitive making it suitable for a broad range of applications.
- Thermal Conductivity Detector (TCD): As analytes elute from the column, they displace the carrier gas, leading to changes in thermal conductivity. Our detector measures this difference in thermal conductivity between the carrier gas “mobile phase “and the analyte molecules.
- Advantages include that it is nondestructive, highly stable, and can detect a wide range of compounds, making it useful for analyzing inorganic and organic compounds.
- Electron Capture Detector (ECD): It can sensitively detect compounds with electron-capturing properties, and depends on its work on the reduction in electric current caused by analyte molecules in a radioactive beta particle-emitting source.
- Mass Spectrometer (MS): Mass spectrometry is detector used in gas chromatography-mass spectrometry systems. It work on both separation and detection as molecules are changed into ionized fragments thus being analyzed based on mass to charge ratio, leading to highly specific identification and quantification of compounds.
- Flame Photometric Detector (FPD): FPD is selective for compounds containing certain elements, such as sulfur or phosphorus. It operates by ionizing analyte molecules in a hydrogen/air flame and detecting the resulting photometric emission specific to the elements of interest.
Conclusion
GC, as well as high-performance liquid chromatography, is a useful and universal analytical instrument. By choosing the injection method, column, detector, and sample pretreatment method, it can be applied to a wide variety of applications. Understanding the system features and appropriate sample treatment can result in a more efficient process. To analyze actual samples, clean-up processes such as dissolution and extraction may be needed. To maintain higher sensitivity, it is important to ensure the instrument’s condition is optimized, and for that, more about troubleshooting.
Stay tuned for the upcoming series on GC troubleshooting and advanced level with pharmacores.
References
https://www.agilent.com/en/product/gas-chromatography-mass-spectrometry-gc-ms/gcms-fundamentals
https://chem.libretexts.org/Bookshelves/Analytical_Chemistry/Physical_Methods_in_Chemistry_and_Nano_Science_(Barron)/03%3A_Principles_of_Gas_Chromatography/3.01%3A_Principles_of_Gas_Chromatographyhttps://scienceinfo.com/gas-chromatography-principle-instrumentation-types/


