What Is the Common Technical Dossier (CTD)
The Common Technical Dossier (CTD), also known as the Common Technical Document, is a globally harmonized format for pharmaceutical regulatory submissions. Developed under the International Council for Harmonisation (ICH), it’s accepted across major markets including the European Union, the United States, and Japan, and increasingly adopted by countries such as Canada, Switzerland, and Australia.
Since its establishment in the early 2000s, it has revolutionized drug registration by standardizing the method of organizing critical data related to quality, safety, and efficacy, thereby enabling companies to submit a single dossier in multiple regions with fewer modifications.
Before the CTD, every country or region had its own specific format for drug registration, leading to duplicated efforts, delays, and inconsistent reviews. The CTD resolved this by offering a harmonized structure that is now widely accepted by regulatory authorities, such as:
- The European Medicines Agency (EMA)
- The U.S. Food and Drug Administration (FDA)
- The Japanese Pharmaceuticals and Medical Devices Agency (PMDA)
- The World Health Organization (WHO) for the prequalification of vaccines
- Numerous national health agencies in Asia, Africa, and Latin America
By implementing the CTD, pharmaceutical companies can prepare a single core dossier that can be submitted to multiple countries with minimal regional adjustments, thereby saving time, money, and administrative burden.
Why the CTD Was Introduced: A Global Problem, One Global Solution
Before the CTD’s introduction in the early 2000s, the pharmaceutical industry faced serious challenges:
- Multiple submission formats for different regulatory bodies
- Increased costs and resource duplication
- Delayed market access
- Misinterpretation or inconsistency in review processes
Recognizing this inefficiency, the ICH established the CTD to:
- Promote regulatory convergence
- Streamline drug development pipelines
- Ensure patient access to safe and effective medicines globally
- Support both innovator companies and generic manufacturers in maintaining compliance
The CTD, therefore, is not just a bureaucratic requirement—it’s a strategic tool for accelerating international product approvals while maintaining rigorous scientific and ethical standards.
Harmonizing Regulatory Submissions Across Borders
A globally aligned structure for presenting new drug authorization materials emerged from cooperative efforts among Europe’s EMA, the FDA in the United States, and Japan’s regulatory body. The goal was to unify the organization, presentation, and readability of application materials for emerging therapies, streamlining preparation, reducing duplication, and enhancing clarity for examiners across major jurisdictions.
These guidelines were formalized by the International Council for Harmonisation in the early 2000s, marking a milestone in regulatory science. Shortly thereafter, use became obligatory in the EU and Japan and was strongly encouraged by U.S. authorities
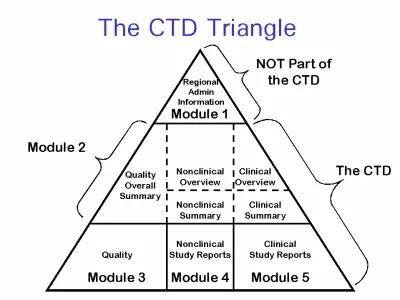
This unified framework is composed of five distinct sections. The first is customized regionally, containing administrative data such as application forms, labeling, and locally specific documents. The subsequent four are standardized globally and consist of summary content, quality data, nonclinical research, and clinical trial findings. Harmonization ensures that life science companies can reuse large portions of content when submitting to multiple territories, significantly improving efficiency and reducing preparation time and cost.

Formatting Best Practices
Globally accepted formatting guidelines ensure consistency and readability. Here are the top recommendations:
- Use clear, legible fonts (e.g., 12‑point serif such as Times New Roman).
- Apply formatting that accommodates both A4 and U.S. Letter.
- Define abbreviations at first mention.
- Number pages sequentially, excluding literature references.
- Include module-specific headers or footers for easy navigation.
- Maintain simple, logical section numbering—avoid unwieldy substructures.
- Cite references in a consistent style, at the end of appropriate sections
How This Structure Benefits Stakeholders
For Developers & Sponsors
A uniform dossier reduces duplication, accelerates preparation for multiple markets, and reduces regulatory costs while enhancing alignment across global filings.
For Reviewers & Regulators
Consistent structure and formatting enable quick navigation, faster scientific assessment, and more efficient cross-validation across files.
Operational Advantages
Electronic layers improve traceability (via XML backbone, checksums, metadata), simplify updates, and expedite amendments or lifecycle management
Evolution & Global Reach
Emerging markets—including Canada, Switzerland, and several Asia-Pacific and Latin American countries—have adopted or adapted the standardized structure for local regulatory systems, broadening its reach beyond the founding ICH regions.
Electronic evolution continues: the latest digital schema, version 4.0, is now supported by the FDA, with eventual mandatory adoption slated beyond 2025. This version enhances flexibility, lifecycle tracking, and metadata features to meet growing regulatory complexity
What Is the CTD Format
The format organizes regulatory submission content into five distinct modules. This standardized layout ensures consistency, transparency, and efficiency in the review process by regulatory agencies across multiple countries.
Whether you’re submitting a New Drug Application (NDA), Marketing Authorization Application (MAA), or Abbreviated New Drug Application (ANDA), adhering to this format is essential for global drug approval.
CTD Format Structure: Overview of the Five Modules
Module 1: Regional Administrative Information
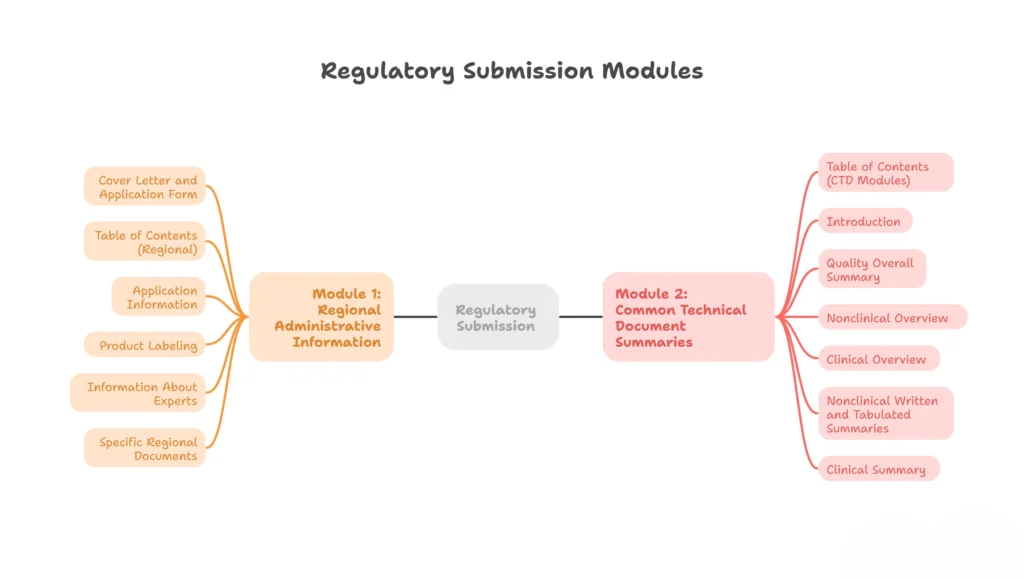
Region-specific content varies by authority (e.g., FDA vs EMA).
Not technically part of the harmonized CTD, but essentially included:
- 1.0 – Cover letter and application form
- 1.1 – Table of contents (regional)
- 1.2 – Application information (including electronic submission info)
- 1.3 – Product labeling
- 1.4 – Information about experts
- 1.5 – Specific regional documents (Risk Management Plan, Orphan designation, etc.)
Module 2 – Common Technical Document Summaries
Summarizes Modules 3–5 for quick regulatory review.
- 2.1 – Table of contents (CTD modules)
- 2.2 – Introduction
- 2.3 – Quality overall summary
- 2.4 – Nonclinical overview
- 2.5 – Clinical overview
- 2.6 – Nonclinical written and tabulated summaries
- 2.7 – Clinical summary
Module 3 – Quality (Chemistry, Manufacturing, and Controls)
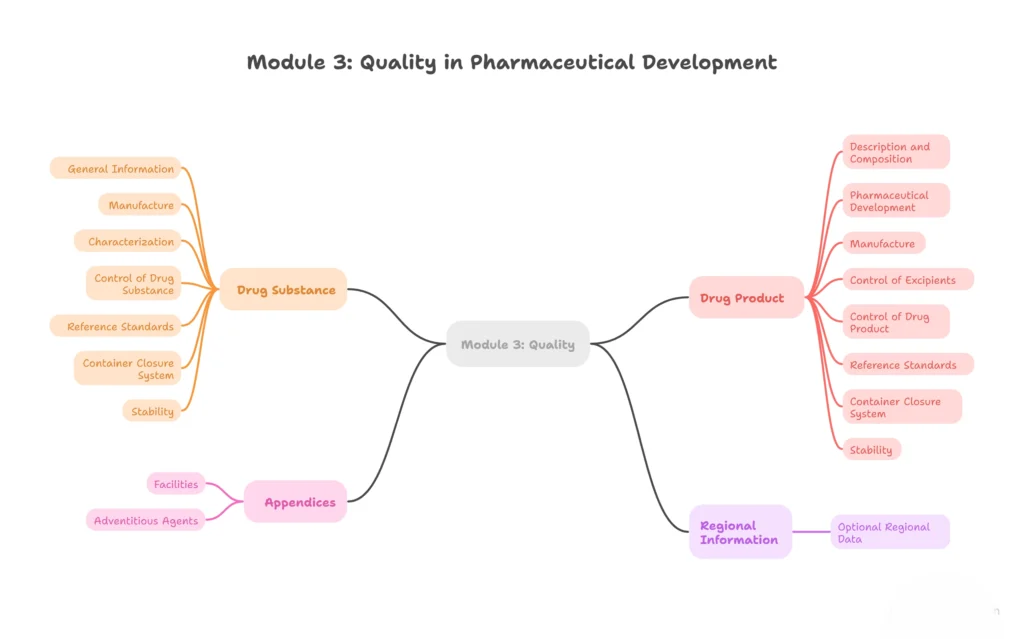
Details related to the drug substance and drug product.
3.1 – Table of contents (Module 3)
3.2 – Body of data
- 3.2.S – Drug substance
- 3.2.S.1 – General information
- 3.2.S.2 – Manufacture
- 3.2.S.3 – Characterization
- 3.2.S.4 – Control of drug substance
- 3.2.S.5 – Reference standards
- 3.2.S.6 – Container closure system
- 3.2.S.7 – Stability
- 3.2.P – Drug product
- 3.2.P.1 – Description and composition
- 3.2.P.2 – Pharmaceutical development
- 3.2.P.3 – Manufacture
- 3.2.P.4 – Control of excipients
- 3.2.P.5 – Control of drug product
- 3.2.P.6 – Reference standards
- 3.2.P.7 – Container closure system
- 3.2.P.8 – Stability
- 3.2.A – Appendices (e.g., facilities, adventitious agents)
- 3.2.R – Regional information (optional)
Module 4 – Nonclinical Study Reports
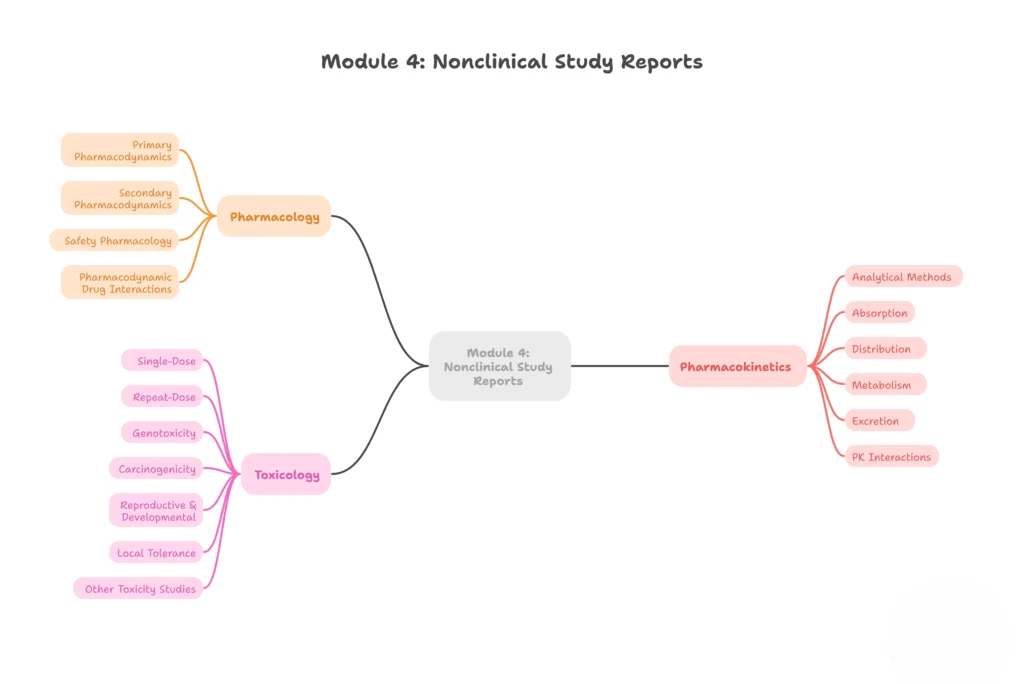
Data from animal studies and laboratory experiments.
4.1 – Table of contents (Module 4)
4.2 – Study reports
- 4.2.1 – Pharmacology
- 4.2.1.1 – Primary pharmacodynamics
- 4.2.1.2 – Secondary pharmacodynamics
- 4.2.1.3 – Safety pharmacology
- 4.2.1.4 – Pharmacodynamic drug interactions
- 4.2.2 – Pharmacokinetics
- 4.2.2.1 – Analytical methods
- 4.2.2.2 – Absorption
- 4.2.2.3 – Distribution
- 4.2.2.4 – Metabolism
- 4.2.2.5 – Excretion
- 4.2.2.6 – PK interactions
- 4.2.3 – Toxicology
- 4.2.3.1 – Single-dose
- 4.2.3.2 – Repeat-dose
- 4.2.3.3 – Genotoxicity
- 4.2.3.4 – Carcinogenicity
- 4.2.3.5 – Reproductive & developmental
- 4.2.3.6 – Local tolerance
- 4.2.3.7 – Other toxicity studies
Module 5 – Clinical Study Reports
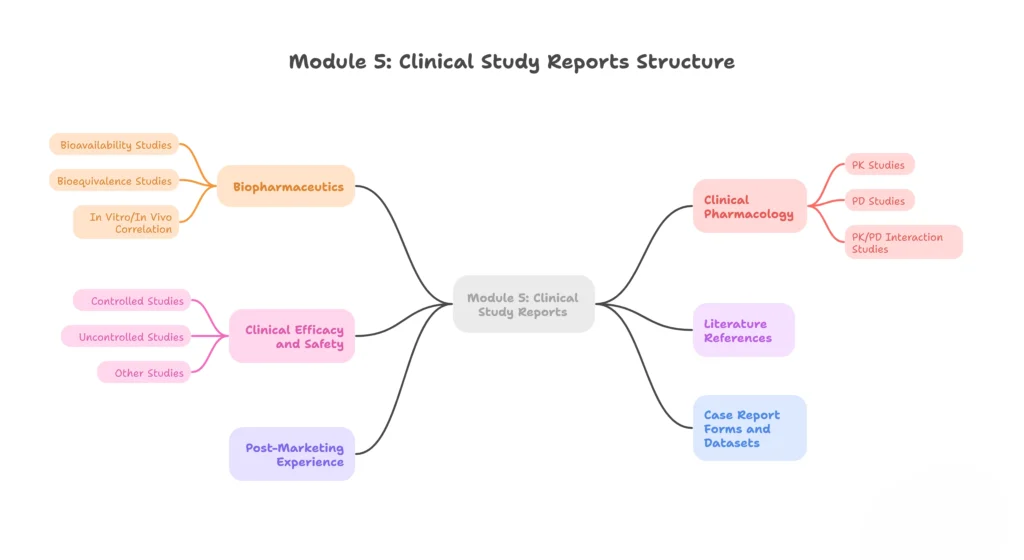
Human clinical trial data: safety, efficacy, and clinical pharmacology.
5.1 – Table of contents (Module 5)
5.2 – Tabular listing of all clinical studies
5.3 – Clinical study reports
- 5.3.1 – Biopharmaceutics
- 5.3.1.1 – Bioavailability studies
- 5.3.1.2 – Bioequivalence studies
- 5.3.1.3 – In vitro/in vivo correlation
- 5.3.2 – Clinical pharmacology
- 5.3.2.1 – PK studies
- 5.3.2.2 – PD studies
- 5.3.2.3 – PK/PD interaction studies
- 5.3.3 – Clinical efficacy and safety
- 5.3.3.1 – Controlled studies
- 5.3.3.2 – Uncontrolled studies
- 5.3.3.3 – Other studies (e.g., special populations)
- 5.3.4 – Literature references
5.4 – Information on post-marketing experience
5.5 – Case report forms and datasets (if required)
Summary Table: CTD Modules
| Module No. | Content | Purpose |
|---|
| 1 | Regional Administrative Info | Country-specific requirements |
| 2 | Summaries | High-level overview of Modules 3–5 |
| 3 | Quality (CMC) | Manufacturing, stability, and control |
| 4 | Nonclinical Reports | Animal/pharmacology safety data |
| 5 | Clinical Reports | Human clinical trial results |
Common Pitfalls in CTD Submissions
| Mistake | How to Avoid It |
|---|---|
| Inconsistent data across modules | Ensure alignment between summaries and full reports |
| Missing rationale for specifications | Include scientific justification |
| Poor document formatting | Follow regional style and font guidelines |
| Errors in eCTD structure | Run full validation before submission |
| Non-harmonized regional modules | Customize Module 1 for each target region |
Summary & Strategic Takeaways
A globally harmonized dossier framework organizes submissions into five clear segments, enabling both local customization and international standardization. Early adoption of electronic submission tools—aligned with ICH and regional guidelines—ensures compliance, efficiency, and reviewer-friendly documentation.
Key strategic considerations:
- Plan globally, adapt locally—use a modular structure to leverage content across multiple authorities.
- Invest in digital infrastructure—e‑submission tools and validation software are vital.
- Adopt formatting best practices to avoid technical rejections.
- Monitor electronic standard updates to stay prepared for evolving submission requirements.
References
International Council for Harmonisation. (n.d.). CTD – Common Technical Document. ICH. https://admin.ich.org/page/ctd
Wikipedia contributors. (2024, June 5). Common Technical Document. Wikipedia. https://en.wikipedia.org/wiki/Common_Technical_Document
Wikipedia contributors. (2024, June 5). Electronic Common Technical Document. Wikipedia. https://en.wikipedia.org/wiki/Electronic_common_technical_document
Therapeutic Goods Administration. (2022). Understanding the Common Technical Document (CTD). Australian Government Department of Health. https://www.tga.gov.au/resources/guidance/understanding-common-technical-document-ctd
World Health Organization. (2021). CTD: Preparation and submission of a vaccine dossier in the format of the Common Technical Document (CTD). WHO. https://extranet.who.int/prequal/vaccines/ctd-preparation-submission
PharmaBoss BD. (2023). Common Technical Document (CTD) Modules. https://pharmabossbd.com/common-technical-document-ctd-modules/
Educo Life Sciences. (2023). A Short Guide to the ICH CTD. https://educolifesciences.com/a-short-guide-to-the-ich-ctd/
Pipeline Pharma. (2023). What is CTD documentation and why is it crucial for pharma companies? https://www.pipelinepharma.com/blog/what-is-ctd-documentation-and-why-is-it-crucial-fo
Pharmacy InfoLine. (2024). Common Technical Document (CTD). https://pharmacyinfoline.com/common-technical-document-ctd/
MedWisdom. (2024). Complete Guide to CTD Dossier. https://medwisdom.in/complete-guide-to-ctd-dossier/
Reddit. (2024). Regulatory and Clinical Writing Discussions. r/RegulatoryClinWriting. https://www.reddit.com/r/RegulatoryClinWriting/