Introduction: Understanding Module 1.3 and Its Importance
The Common Technical Document (CTD) is a globally accepted standard for submitting applications to regulatory authorities for the registration of pharmaceutical products. Among its five modules, Module 1 is region-specific and includes administrative and product-specific information. Within this, Module 1.3 is crucial as it covers Labeling and Product Information — including package inserts, summary of product characteristics (SmPC), and labeling
These documents are not merely formalities. They ensure that healthcare professionals and patients receive consistent, accurate, and clear information about the drug. This article explores the requirements, regional variations, and the critical role accurate labeling plays in patient safety
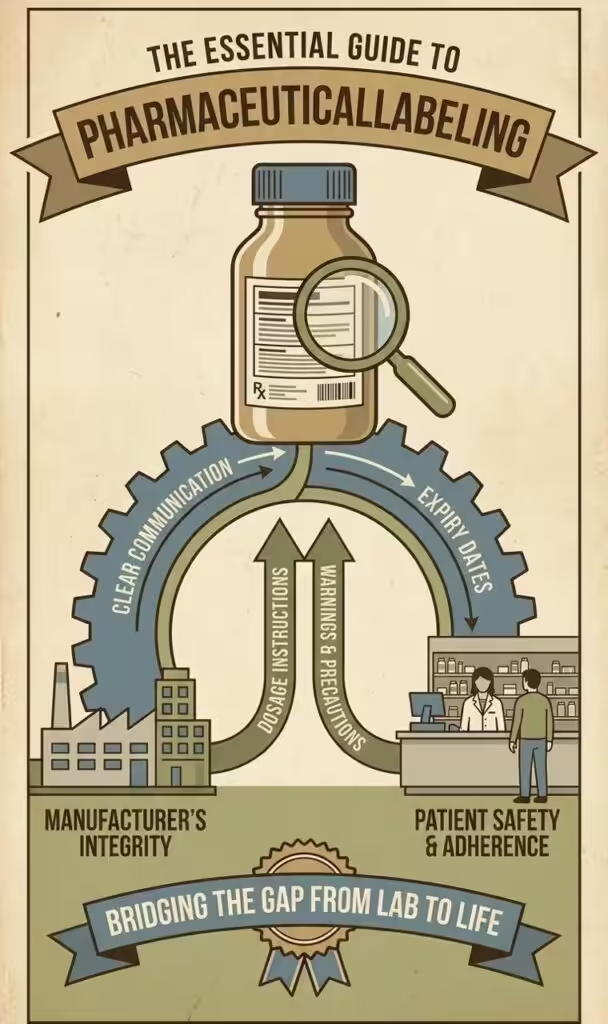
What Is CTD Module 1.3
Module 1.3 focuses on product labeling and related documentation required by different regulatory bodies. It typically includes:
- Package Inserts (PIs)
- Summary of Product Characteristics (SmPC)
- Patient Information Leaflets (PILs)
- Outer and Inner Packaging Labels
- Mock-ups and Specimens
- Regional Labeling Forms (where required)
Each of these documents serves a specific function in communicating essential information about a medicinal product
Requirements for Package Inserts and Patient Information Leaflets (PILs)
Package Inserts (PI)
Package inserts are primarily intended for healthcare professionals. They contain comprehensive details including:
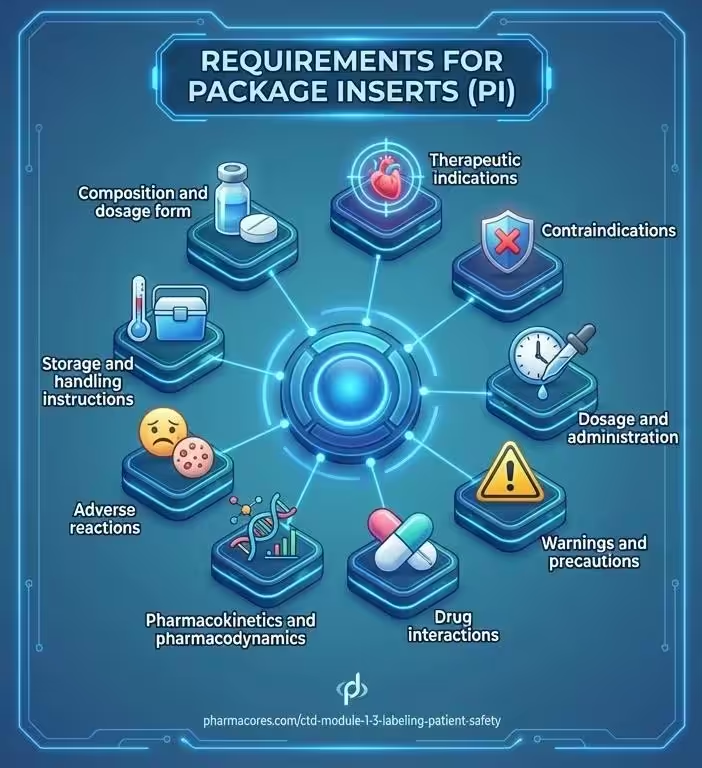
- Composition and dosage form
- Therapeutic indications
- Contraindications
- Dosage and administration
- Warnings and precautions
- Drug interactions
- Pharmacokinetics and pharmacodynamics
- Adverse reactions
- Storage and handling instructions
These inserts must be written in technical language, be scientifically accurate, and conform to the regulatory formatting guidelines of the region.
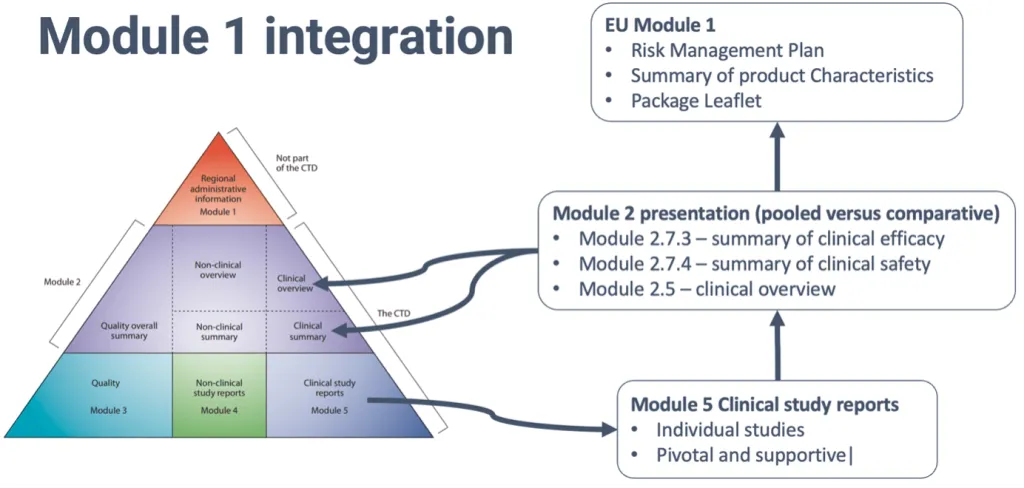
CTD Module 1: Administrative and Regional Information – The Foundation of Regulatory Submissions
Patient Information Leaflets (PILs)
PILs are crafted for end-users for the patients. These must be written in simple, non-technical language, addressing:

- What the medicine is for
- How to take it
- Possible side effects
- When not to use the medicine
- How to store it
- Safety instructions (e.g., use during pregnancy)
They must be readability tested in many regions to ensure clarity and accessibility, especially for people with low health literacy.

Summary of Product Characteristics (SmPC): The Regulatory Backbone
The SmPC is a regulatory document required primarily in European countries. It serves as a formal agreement between the regulatory authority and the marketing authorization holder regarding the use of a medicine.
Key Sections of the SmPC:
- Name, strength, and dosage form
- Clinical particulars (e.g., indications, contraindications, special warnings)
- Pharmacological properties
- Pharmaceutical particulars (e.g., shelf life, storage)
- Marketing authorization details
The SmPC forms the basis for the package insert and PIL, ensuring consistency in the message across professional and patient documents.
Labeling Requirements: Outer and Inner Packaging
Both the primary (inner) and secondary (outer) labels must contain clear, legible information including:
- Brand name and generic name
- Dosage strength and form
- Batch number
- Expiry date
- Storage instructions
- Manufacturer and distributor details
- Regulatory symbols or warnings (e.g., “Rx only” or hazard icons)
These labels are strictly regulated, as they are often the primary source of information for healthcare providers and patients during storage and administration.
Global and Regional Differences in Labeling Requirements
United States (FDA)
- FDA requires submission of FDA Form 356h, a comprehensive package insert for healthcare professionals, and medication guides for patients.
- The Physician Labeling Rule (PLR) governs the structure of labeling.
- Requires a DailyMed submission for public access to labeling.
🇪🇺 European Union (EMA)
- Requires SmPC, PIL, and labeling texts in multiple languages.
- Readability testing of PILs is mandatory.
- QRD templates are used to standardize format and structure.
🇯🇵 Japan (PMDA)
- Uses the Package Insert (PI) structure, but places strong emphasis on adverse event reporting.
- Labeling must be compliant with Japanese Pharmacopoeia standards.
🇨🇦 Canada (Health Canada)
- Requires Product Monograph (PM), which is similar to SmPC but includes a Consumer Information Section.
- Both English and French versions are mandatory.
🇮🇳 India (CDSCO)
- Requires package insert and labeling in accordance with Drugs and Cosmetics Rules.
- Emphasis on drug schedule category (e.g., Schedule H, X).
- Language requirements are less standardized compared to EU or US.
The Impact of Accurate Labeling on Patient Safety
Accurate and clear labeling is essential in ensuring patient safety across healthcare systems. One of the most critical roles of proper labeling is in preventing medication errors. Mislabeling or unclear instructions can lead to the administration of the wrong drug, incorrect dosage or frequency, and overlooked contraindications. According to the World Health Organization (WHO), medication errors are among the leading causes of avoidable harm worldwide. Proper labeling serves as a frontline defense against such risks, providing healthcare professionals with the information needed to prescribe and dispense medications safely.

Informed Patient Decisions
When patients have access to well-labeled medications, they are more empowered to make informed decisions about their treatment. Labels that clearly explain what the drug is used for, how it should be taken, and what side effects to monitor, help improve treatment adherence. Patients who understand their medications are more likely to follow prescribed regimens and report adverse effects promptly. This not only enhances individual outcomes but also supports broader public health efforts by ensuring treatments are used safely and effectively.
Warnings and Contraindications
Proper labeling plays a crucial role in highlighting warnings and contraindications. When labeling is comprehensive and accurate, it helps prevent allergic reactions, dangerous drug interactions, and inappropriate use in patients with certain conditions, such as pregnancy or liver disease. For instance, the omission of a warning about a drug’s teratogenic effects could have devastating consequences for pregnant patients. Clear communication of such risks is therefore vital to safeguarding vulnerable populations.
Ensuring Equity Across Populations
Labeling also serves as a tool for health equity. In multi-ethnic countries and during global product launches, multilingual and culturally sensitive labeling ensures that all patient populations can safely understand and use medications. This approach supports inclusive healthcare delivery, helping to overcome language and literacy barriers that might otherwise lead to misuse or non-compliance.
Labeling in the Digital Age: Trends and Innovations
With the advancement of technology, pharmaceutical labeling is undergoing significant transformation. Electronic labeling (eLabeling) is becoming increasingly common, offering benefits such as real-time updates to product information and improved accessibility via QR codes or dedicated apps.
In addition to improving patient access to information, eLabeling reduces the environmental impact by minimizing the use of paper inserts. Regulatory authorities like the European Medicines Agency (EMA) and the U.S. Food and Drug Administration (FDA) are now supporting initiatives such as electronic Product Information (ePI), further validating the move toward digital formats.
Another innovation in this space includes the use of smart labels and track-and-trace technology. Radio-frequency identification (RFID) tags, barcodes, and tamper-evident packaging are being increasingly used to not only enhance patient safety but also improve supply chain security. These technologies play a pivotal role in combating the global challenge of counterfeit drugs, ensuring that medications reaching patients are genuine and unaltered.
Challenges in Preparing Module 1.3 Documents
Despite its importance, preparing the labeling documents required in CTD Module 1.3 poses several challenges. Language and translation issues are a major concern, as poor translation of Patient Information Leaflets (PILs) or Summaries of Product Characteristics (SmPCs) can lead to misinterpretation, putting patient safety at risk and exposing companies to legal consequences. Furthermore, keeping information up to date is a continuous requirement. Marketing Authorization Holders (MAHs) must ensure that labeling reflects the latest safety data, updates in indications, and regulatory changes to maintain compliance and protect patients.
Another major challenge lies in balancing technical accuracy with readability. Labels that are too technical may be incomprehensible to the average patient, while overly simplified language risks omitting critical safety details. Striking the right balance between clarity and comprehensiveness is essential to ensure that both healthcare professionals and patients can use the information effectively.
What Are IND Ethics & Safety Requirements? FDA Clinical Trial Guide
Conclusion: Labeling in CTD Module 1.3 Is More Than a Compliance Task
Ultimately, the preparation and maintenance of labeling within CTD Module 1.3 go far beyond regulatory compliance. These documents—ranging from package inserts and SmPCs to PILs and multilingual labels—form the foundation of safe, effective, and ethical medication use. Accurate and accessible labeling ensures that healthcare providers and patients alike are equipped with the knowledge necessary for proper drug administration.
Given the diverse regulatory landscapes and populations served worldwide, it is essential to consider regional requirements and cultural contexts when developing labeling. Maintaining consistency, clarity, and accuracy is not just a professional or legal duty—it is a moral obligation rooted in patient safety. In an increasingly global and digital healthcare environment, effective labeling remains a cornerstone of responsible pharmaceutical practice.
References
- European Medicines Agency (EMA). (2023). Guideline on the readability of the label and package leaflet of medicinal products for human use. https://www.ema.europa.eu
- U.S. Food and Drug Administration (FDA). (2020). Labeling for Human Prescription Drug and Biological Products – Implementing the PLR Content and Format Requirements. https://www.fda.gov/media/71866/download
- International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH). (2003). M4: Organisation of the Common Technical Document for the Registration of Pharmaceuticals for Human Use. https://www.ich.org/page/ctd
- World Health Organization (WHO). (2016). Medication errors: Technical series on safer primary care. https://www.who.int/publications/i/item/9789241511643
- Health Canada. (2022). Guidance document: Product Monograph. https://www.canada.ca/en/health-canada/services/drugs-health-products/drug-products/applications-submissions/guidance-documents/product-monograph.html
- Pharmaceuticals and Medical Devices Agency (PMDA, Japan). (2021). Package Insert Notifications and Guidelines. https://www.pmda.go.jp
- Central Drugs Standard Control Organization (CDSCO, India). (2016). Drugs and Cosmetics Rules, 1945 – Labeling Requirements. https://cdsco.gov.in
- European Medicines Agency (EMA). (2021). Electronic Product Information (ePI) initiative. https://www.ema.europa.eu/en/human-regulatory/overview/electronic-product-information