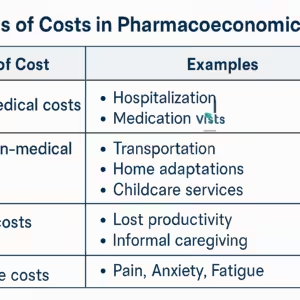
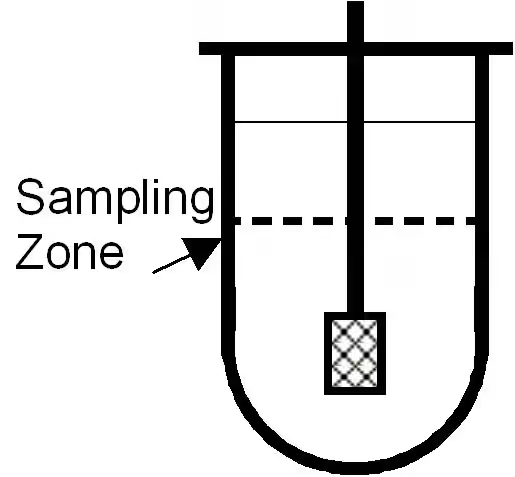
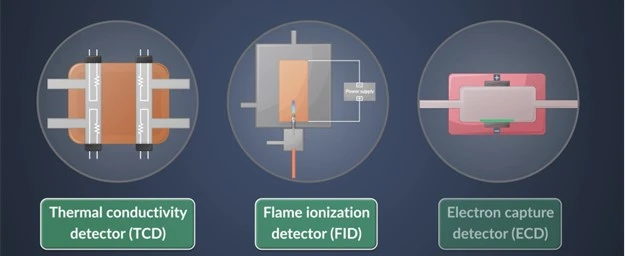
Filter posts by category
- All
- Advanced Drug Delivery Systems Engineering
- Analytical Method Development
- Basics of Pharmacovigilance
- Bioequivalence
- Drug Discovery
- Excipients & Compatibilities
- Formulation Development
- Good Manufacturing Practices (GMP)
- Hazard Handling and Safety Measures
- Operational Excellence in Pharmaceutical Industry
- Pharmaceutical Innovation
- Pharmaceutical Microbiology
- Pharmaceutical Quality
- Pharmaceutical R&D
- PharmaCoresGeneralTopics
- Pharmacovigilance
- Production
- Production Scheduling
- Quality Assurance
- Quality Control
- Regulatory Affairs
- Uncategorized