Introduction to Dosage Forms and Bioequivalence
Drugs are formulated in various dosage forms, including:
- Solids (e.g., tablets, capsules)
- Semisolids (e.g., ointments, creams)
- Liquids (e.g., suspensions, emulsions)
These are developed to produce either systemic or localized therapeutic effects.
Drug formulations function as delivery systems, ensuring that the active pharmaceutical ingredient (API) reaches the target site for optimal therapeutic effect. To achieve this, biopharmaceutic and pharmacokinetic (PK) studies are essential in understanding how drug properties influence their performance in the body.
Need a refresher on the fundamentals before diving deeper?
Explore Part 1 of this series to understand the core concepts of Bioavailability and Bioequivalence, including key definitions and the science behind drug absorption.
👉 Start with the Bioavailability and Bioequivalence in Pharmaceuticals: A Comprehensive Introduction
Understanding Biopharmaceutics and Pharmacokinetics
Biopharmaceutics and pharmacokinetic (PK) studies play a crucial role in understanding how the physicochemical properties of a drug influence its pharmacologic and clinical effects. Ultimately, such studies contribute to the development of safer and more effective medications.
Biopharmaceutics evaluates how a drug’s physicochemical characteristics, dosage form, and route of administration influence its absorption into systemic circulation. It focuses on the rate and extent of absorption, crucial for determining therapeutic efficacy.
The interaction between the drug and its formulation is key in absorption and distribution, forming the basis for bioavailability (BA) and bioequivalence (BE) evaluations.
Importance in Drug Development
This process is a sequence of events that occur before the drug can exert its therapeutic effect. Understanding these biopharmaceutics and PK parameters is essential in designing and interpreting the bioequivalence ( BE) studies, which aim to evaluate the bioavailability(BA) between different drug formulations.
- BA assesses how much and how quickly a drug reaches systemic circulation.
- BE studies determine if two drug formulations offer similar bioavailability.
- Understanding these parameters helps ensure safe, effective, and interchangeable drug products.
The Drug Journey: From Administration to Action
The journey of a drug from administration to its therapeutic effect follows a well-defined sequence. Firstly, the patient takes the drug through the specified route, oral, intravenous, subcutaneous, or transdermal administration. Then, the drug is released from its dosage form in a controlled and predictable manner. Following release, a portion of the drug is absorbed either into local tissue (targeted effect) or the systemic circulation (broader action). The extent and rate of absorption depend on the drug’s formulation and route of administration. Finally, the drug reaches its site of action, where it interacts with biological receptors to produce a pharmacodynamic (PD) effect
- Administration: Drug enters the body (oral, IV, subcutaneous, transdermal).
- Release: The Drug is liberated from the dosage form.
- Absorption: The Drug enters the tissue or systemic circulation.
- Action: The Drug reaches the target site and produces pharmacodynamic effects.
BA, which measures the rate and extent to which a drug reaches systemic circulation in its active form, can vary even between drug products containing the same drug and administered through the same route. Differences in BA can lead to variations in therapeutic effectiveness, meaning one formulation may be more effective than another, even if they contain the same amount of active pharmaceutical ingredient (API).
Several factors influence BA, including
- The drug’s molecular characteristics
- Its delivery method
- The formulation of its dosage form.
- These elements collectively determine whether the drug will achieve its intended therapeutic effect, cause toxicity, or have little to no impact
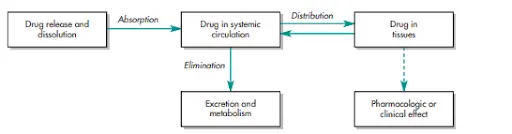
Pharmacokinetics (PK) and ADME
The PKs field merges experimental techniques, like biological sampling and analytical methods, with theoretical modeling to predict drug behavior in the body. The interplay between drug absorption, distribution, metabolism, and excretion (ADME) is crucial for optimizing drug therapy and ensuring its safety and effectiveness.
As previously described, PK studies rely on biological fluid sampling to estimate drug concentration change over time. Since direct measurement at the receptor site is impractical, so, plasma drug concentration is used instead, assuming the equilibrium between plasma and tissue levels.
Why Bioequivalence (BE) Matters
For immediate-release oral dosage forms, BE is assessed through:
- Clinical PK studies
- In vitro dissolution tests
BE ensures that two drug products containing the same active ingredient exhibit similar rate and extent of absorption (i.e., are considered bioequivalent) when administered in the same molar dose. This concept is fundamental for generic drug approval, where BE studies confirm that the generic performs identically to the reference product, maintaining safety and efficacy without requiring additional clinical trials.
- Contain the same API
- Deliver it at the same rate and extent
- Provide equivalent therapeutic outcomes
With a clear understanding of the terminology and importance of BE and BA, let’s now dive into the practical aspects of how bioequivalence is assessed by examining a study protocol in detail.
Transition to Practice: Components of a BE Study Protocol
Bioequivalence Study Protocol Elements
1.1 Study Objective
To compare the bioavailability of a test product versus a reference product in orally administered solid dosage forms.
1.2. Study design
The basic design of a bioequivalence study is shaped by several key factors, including the:
- Specific scientific questions and study objectives,
- The characteristics of the reference material and dosage form,
- The availability of analytical techniques, the pharmacokinetic and pharmacodynamic properties of the drug,
- The administration route, and
- The ethical and benefit–risk considerations involved in human testing.
An appropriate study design and thorough statistical analysis are key factors in establishing BE. A blood concentration-time profile is influenced by various subject-related factors, such as age, sex, health status, dietary habits, body weight, and disease state, as well as experimental parameters like study design, dosing time, sampling schedule, analytical techniques, and pharmacokinetic modeling. And so, careful consideration of these variables is essential in the study design process.
1.2.1. Experimental design
1.2.1.1. Parallel design:
Where two formulations are given to two separate groups of volunteers, ensuring randomization, which is applied to minimize bias in administration. However, a key drawback of this design is its inability to correct the inter-subject variability. Studies have consistently shown that differences between individuals often exceed variations observed between formulations. A nonreplicate, parallel study design is generally used for drug products containing compounds with prolonged elimination half-lives or formulations like depot injections, where drug release occurs gradually over weeks or months.
Blood sampling must be conducted over a sufficient duration of approximately 2–3 days to ensure complete gastrointestinal transit and absorption of the drug substance. However, this design is not suitable for drugs exhibiting a high intrasubject variability in distribution and clearance.
1.2.1.2. Cross-over design:
It is favored in bioavailability and bioequivalence studies as it effectively minimizes the impact of inter-subject variability. This is guaranteed by using each subject as his/her control. A full crossover design is commonly utilized, ensuring that each subject receives both the test formulation and the reference product. Generally, the highest strength to be marketed is the strength to be used in the BE study. Single-dose studies offer the most sensitive conditions for identifying subject-to-subject variations in both the rate and extent of drug absorption.
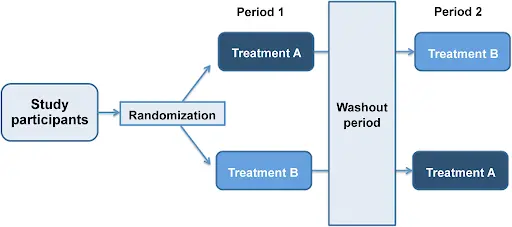
Fig.2. Illustration of crossover design study
1.2.1.3. Multiple dose (steady-state) design:
Multiple doses of the same drug are administered consecutively to achieve steady-state plasma drug concentrations. The multiple-dose study follows a steady-state, randomized, two-treatment, two-way crossover design, comparing equal doses of the test and reference formulations in healthy adult subjects.
Each participant receives either the test or reference product, with a designated ‘washout’ period between treatments. A multiple-dose study may be required to determine the bioavailability of a drug product in the following circumstances:
- There is a difference in the rate of absorption, but not in the extent of absorption.
- There is excessive variability in bioavailability from subject to subject.
- The concentration of the active drug ingredient or therapeutic moiety, or its metabolite(s), in the blood resulting from a single dose is too low for accurate determination by the analytical method.
- The drug product is an extended-release dosage form
The multiple-dose crossover method for bioequivalence assessment has several drawbacks.
- First, achieving steady-state conditions extends study duration, leading to increased clinical costs and a higher risk of subject dropout.
- Second, additional plasma samples are required to confirm steady-state and accurately characterize the plasma concentration-time profile.
- Third, since Cav is primarily influenced by drug dose and dosing intervals, systemic drug availability is more critical than absorption rate, meaning minor differences in absorption may go undetected in steady-state comparisons. It is important to know that the average drug concentration at steady state is not the mean value of the maximum and the minimum plasma concentration.
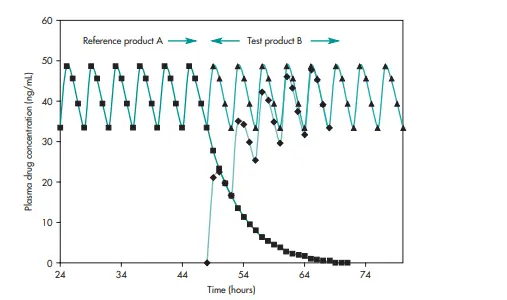
Fig.3. Multiple-dose bioequivalence study conducted in patients where the bioequivalence is assessed by comparing the steady-state plasma concentration-time profiles of the reference drug product (A) and the test drug product (B) following administration
1.2.1.4 Clinical Endpoint Study:
Typically follows a randomized, double-blind, placebo-controlled, parallel design, while comparing test product, reference product, and placebo in patient populations. A placebo arm is included to confirm treatment efficacy by ensuring responses exceed the no-effect portion of the dose-response curve, demonstrating that the study has sufficient sensitivity to detect clinical effects within the enrolled patients. The primary bioequivalence analysis is conducted by comparing the proportion of patients in the test and reference treatment groups who achieve a ‘therapeutic cure’ by the end of the study.
1.2.2. Washout period
In crossover trials, a washout period is implemented to reduce potential carryover effects between treatment phases. This phase must be sufficiently long to ensure the complete elimination of the first intervention’s influence before the next treatment begins. Treatment periods should be spaced out by an adequate washout duration, typically at least five elimination half-lives, to prevent carryover effects. It is essential to ensure that the washout period is sufficiently long, as carryover effects may not be easily identified. The required washout period is determined by the drug’s half-life and dosage.
1.2.3. Drug products
- Test product(s): Test products refer to new drug formulations developed by pharmaceutical technologists or modified dosage forms of existing drugs. These formulations are compared against a reference standard recognized by the FDA to obtain approval for market entry.
- Reference standard: A chemical or generic drug product must be compared to a standard dosage form to evaluate its in vivo performance. Generally, FDA designates the innovator drug product—the originally approved formulation—as the reference standard. In cases where multiple manufacturers hold approval for the same drug, any of the permitted formulations may be used as a reference. However, the FDA may require a single designated reference product to ensure consistency and comparability in bioequivalence assessments.
1.2.4. Route of administration
Bioavailability studies primarily focus on orally administered dosage forms, but formulations delivered via buccal, transdermal, and intramuscular routes also require evaluation of their biological performance. The therapeutic efficacy of these dosage forms depends on the rate and extent of drug absorption. Oral dosage forms tend to exhibit significant inter-subject and intra-subject variability, which influences their performance. All extravascular administered formulations necessitate bioavailability assessments to ensure consistent therapeutic effects.
1.2.5. Dosage regimen
For bioequivalence assessment,
- Single-dose studies are generally sufficient, as the relative bioavailability of most tablets and capsules can be effectively determined from a single-dose evaluation, which often predicts multiple-dose performance. Dosage forms intended for single-dose therapeutic effects, such as analgesics, typically require only single-dose studies
- Multiple-dose studies are necessary for certain formulations designed for modified drug release, including time-release products, enteric-coated preparations, and some intramuscular injections. Moreover, drugs undergoing first-pass metabolism require multiple-dose evaluation to ensure accurate bioavailability assessment. The selection of an appropriate study design is critical, as an improper design may result in insufficient or inaccurate data collection. Furthermore, special dosage regimens like loading doses or twice-daily dosing may also warrant a multiple-dose study.
1.2.6. Frequency and duration of sampling
The sampling schedule in a BE study must comprehensively capture the concentration-time curve.
- This includes a pre-dose sample, measurements during the absorption phase, frequent sampling around the anticipated time to maximum observed concentration (Tmax), and sufficient data points to ensure an accurate estimation of the extent of exposure.
- This is typically achieved when AUC(0–t) accounts for at least 80% of AUC(0–inf).
- The sampling period should generally extend to at least three times the terminal elimination half-life of the drug unless an appropriate truncated AUC, such as AUC(0–72h), is utilized. To facilitate the calculation of key pharmacokinetic parameters, a sufficient number of samples must be collected per subject in each study period, ensuring coverage across all phases of drug disposition.
- In multiple-dose studies, the pre-dose sample should be collected within 5 minutes before dosing to ensure accurate baseline measurement. The final sample is recommended to be taken within 10 minutes of the nominal dosing interval to precisely determine AUC(0–τss).
1.2.7. Randomization of drug administration
Bias reduction relies on proper study design, methodology, and analysis, as analytical methods cannot correct flaws stemming from poor study design.
Several techniques help minimize bias, with randomization being a key approach. Randomization ensures that samples are assigned to groups purely by chance, distributing potential confounders evenly across study arms to reduce bias.
- In simple randomization, each individual has an equal and independent chance of selection, such as drawing numbers from a hat. While this method is straightforward, its drawback is that early study termination may result in uneven group sizes at different time points.
- In block randomization, on the other hand, subjects are placed into small predefined groups (blocks), typically ranging from 4 to 20 participants. This approach maintains balanced group sizes throughout the study, ensuring equal comparisons and more valid conclusions, even if the study is stopped prematurely
1.2.8. Single-versus multiple-dose study design
The choice between single-dose and multiple-dose studies for bioavailability assessment depends on several factors:
- The drug’s pharmacokinetics
- therapeutic application
- regulatory requirements.
- Single-dose studies are generally preferred for BE assessments intended for immediate-release formulations, as they effectively characterize the absorption rate and extent of the drug without any complexities in the steady-state pharmacokinetics. They are often sufficient when the drug exhibits linear pharmacokinetics, where absorption and elimination rates remain consistent across administered doses.
- Multiple-dose studies are necessary for drugs with nonlinear pharmacokinetics, undergoing significant first-pass metabolism, or for modified-release formulations (e.g., controlled-release tablets, transdermal patches). These studies help evaluate steady-state concentrations, which are crucial for understanding long-term drug exposure and therapeutic effect.
References:
- Biopharmaceutics and Pharmacokinetics – V. Venkateswarlu, University College of Pharmaceutical Sciences, Kakatiya University, Warangal, Andhra Pradesh.
- ICH Guidelines – European Medicines Agency


