Introduction
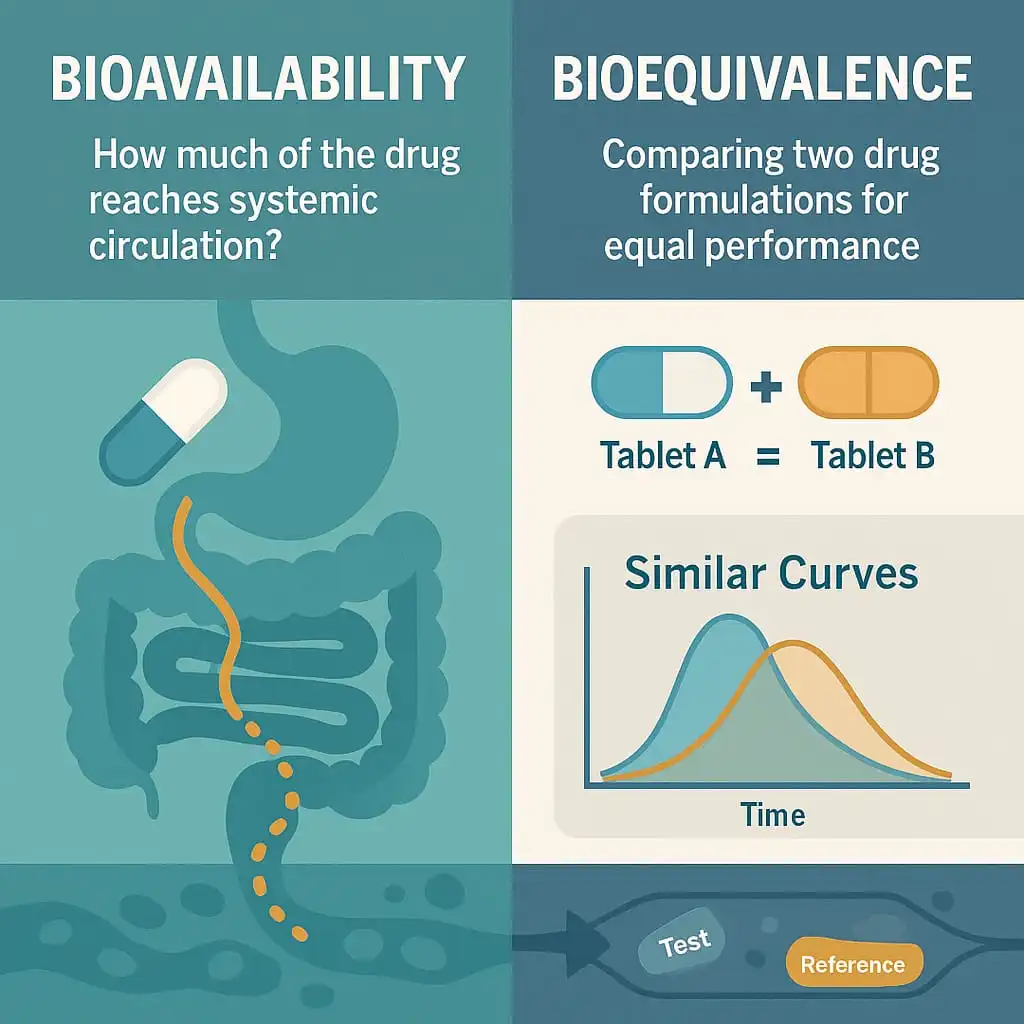
Bioavailability & Bioequivalence studies are crucial in the pharmaceutical industry. The prime target of most oral dosage forms is to deliver the drugs into the bloodstream, ready to be distributed to where it is needed in the body to have their effect (site of action). The therapeutic effect of a drug depends on achieving and maintaining a suitable concentration at its site of action.
For systemically active drugs, a dynamic equilibrium exists between the drug concentration in the blood and its concentration at the target site, which predicts a linear relationship. This means that the drug level at its site of action can be estimated based on its concentration in the blood. Drug concentration in plasma water (i.e., protein-free plasma) is a more precise indicator than total plasma concentration, but measuring it is a complicated process.
Understanding Drug Concentration and Plasma Levels
Despite this assumption, a correlation between plasma concentration and clinical effects is not always guaranteed.
Various factors influence plasma drug levels, including:
- The proportion of the administered dose that enters systemic circulation
- The rate of distribution to tissues and fluids
- The rate of elimination.
Therefore, plasma drug concentration alone may not always reliably predict therapeutic response, unless consistent correlations have been established through research.
Intravenous vs. Extravascular Drug Administration
Intravenous (IV) Bolus Injection
In the case of an intravenous (IV) bolus injection, the entire drug dose immediately enters the bloodstream, bypassing absorption barriers. Once in circulation, the drug undergoes processes such as protein binding, distribution throughout the body, metabolism (mainly in the liver), and elimination via renal excretion or other pathways. The drug exerts its pharmacological effects as long as its concentration in the blood remains above the minimum therapeutic level, which is required for drug efficacy.
Extravascular Administration (e.g., oral, IM, SC)
For drugs administered extravascularly (e.g., orally, intramuscularly, or subcutaneously), additional factors influence the drug levels in blood as:
- The rate at which the drug is released from its dosage form
- The efficiency of its absorption at the site of administration.
- These aspects determine the rate and extent to which the drug reaches systemic circulation, ultimately affecting its therapeutic effect.
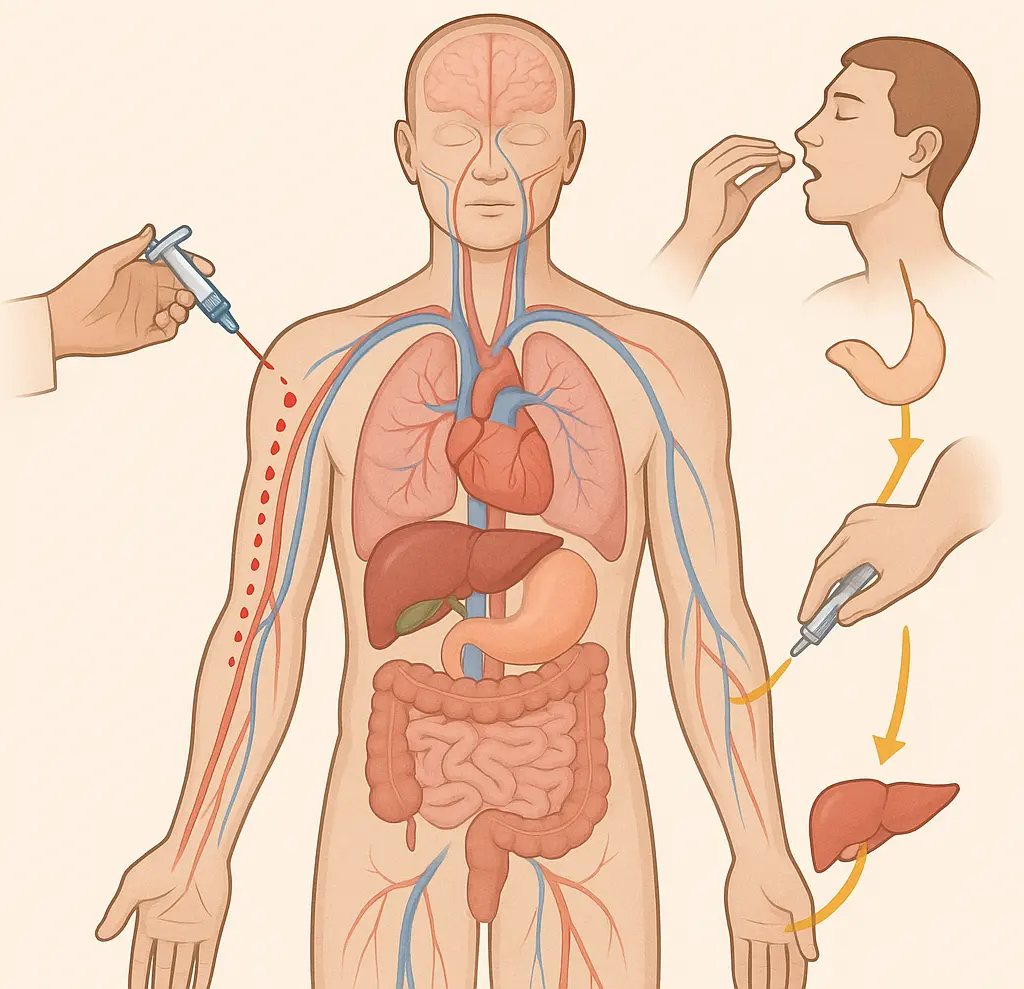
Fig. 1 shows Intravenous vs. Extravascular Drug Administration
Factors Influencing Bioavailability
Factors influencing bioavailability include:
- Drug’s formulation
- Rate of absorption
- First-pass metabolism (especially for orally administered drugs)
- Food or drug interactions
Understanding bioavailability helps in optimizing dosing regimens to ensure effective treatment while minimizing variability in drug response.
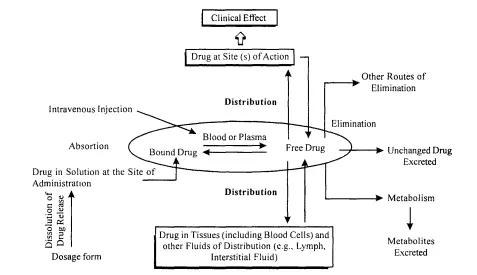
Fig. 2 shows the factors influencing the concentration of the drug in blood
Types of Studies
1. Evaluation of a New Formulation (First Type)
- Understanding how the body absorbs, distributes, and eliminates a new drug (pharmacokinetics) when given through various routes.
Aim of this study
This knowledge helps in designing a suitable dosage schedule. The drug is then prepared in the most appropriate form for its intended use & its bioavailability is measured.
2. Bioequivalence Studies(Second Type)
- Comparing a new drug formulation to a reference standard. comparing a new drug formulation to a reference standard (a version of the drug already known for its safety & efficacy to patients).
- New drug products must meet strict safety & effectiveness standards before they can be marketed; therefore, approval is granted when a drug product demonstrates bioequivalence to a safe and effective reference product.
When are two products considered to be bioequivalent?
Two products are considered to be bioequivalent when they are equivalent in the rate and extent to which the active pharmaceutical ingredient (API) becomes available at the site(s) of drug action. In other words, if the drug concentration-time profiles of the two products are similar and show no significant difference in their therapeutic or adverse effects.
Both bioavailability & bioequivalence relate to how a drug is released from a product and then absorbed into the body’s systemic circulation.
Key Terms in Bioavailability and Bioequivalence
To ensure a clear understanding of bioavailability and bioequivalence guidelines, let’s start by defining some of the terms you will encounter:
Bioavailability (BA) is defined as the rate and extent to which a drug is absorbed from its dosage form.
IV formulation = 100% bioavailability
Oral formulation = less than 100% bioavailability
Absolute Bioavailability of a drug is measured by comparing the individual BA after an oral & IV bolus injection (i.e., the actual amount of drug released from the product that enters the bloodstream).
Relative bioavailability is the fraction of an orally administered dose that is absorbed into the systemic circulation in comparison with a marketed reference (FDA clinically proven standard dose of the same API).
Understanding Pharmacokinetic Parameters
Pharmacokinetic parameters are measurements that tell us what the body does to a drug. We find these measurements by looking at the drug & its breakdown products (metabolites) in body fluids such as plasma, serum or urine. These measurements give us a general idea of how the drug undergoes absorption (influenced by the route of administration), distribution (distributed to various tissues), metabolism (metabolised or broken down) & excretion (eliminated from the body).
Absorption, distribution, metabolism and excretion of drugs are the processes in which a drug moves in the body at diverse rates. The relative rates of these “ADME” processes regulate the time profile of the drug in our body.
In pharmacokinetics, the concentration of a drug in plasma is determined over time following its administration. When a drug enters the systemic circulation, its plasma concentration gradually rises as long as the absorption rate surpasses the elimination rate. This increasing drug level in the plasma leads to a progressive rise in the rate of drug elimination. As time progresses, the absorption rate slows down while the elimination rate accelerates. Eventually, a specific point is reached when both rates equal each other. The rate of these processes varies over time as the amount of drug in the body changes.
Explanation of Basic Pharmacokinetic Parameters
For better understanding, we will explain the terminology of the Basic pharmacokinetic parameters obtained from plasma concentrations after an administration of a drug:
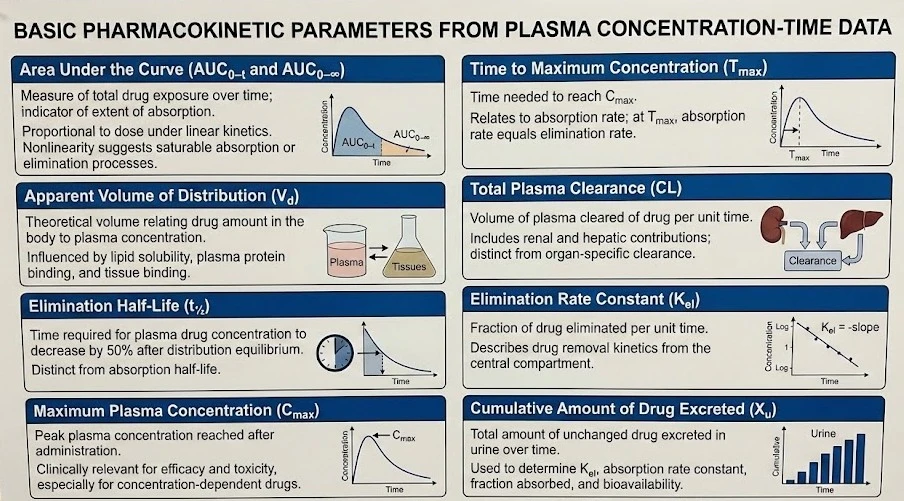
Area Under Curve (AUC)
It is the measure of the concentration of a drug in the blood plasma as a function of time from time zero to time t (AUC₀₋ₜ), where t is the last time point with a measurable concentration, i.e., a measure of the extent of absorption. The units of AUC are concentration-time/volume, µg·hr/ml.
- For most drugs, AUC is directly proportional to the administered dose.
- The area under the curve (AUC) remains unaffected by the drug’s route of administration and the mechanisms of elimination, provided that the elimination process remains stable.
- If the relationship between the administered dose and the area under the curve shows nonlinearity, it typically suggests the presence of saturable absorption and/or elimination kinetics. In cases where AUC does not increase proportionally with the dose, accurate assessment of the drug’s bioavailability becomes challenging.
AUC (₀₋∞)
It is the area under the concentration vs. time curve extrapolated to infinity.
Apparent Volume of distribution (Vd)
It is the theoretical volume that would be necessary to contain the total amount of an administered drug at the same concentration that is observed in the blood plasma. The volume of distribution of most drugs is typically equal to or smaller than the body mass, though some drugs have a Vd that exceeds this significantly.
- Lipid-soluble drugs with minimal plasma protein binding tend to have a high Vd, leading to greater concentration in the bloodstream. In contrast, highly polar drugs that struggle to penetrate tissues and are strongly bound to plasma proteins have a lower Vd.
- Drugs that bind to tissues also exhibit a high Vd due to their low initial plasma concentration. However, the interplay between tissue penetration, lipid solubility, and protein binding in many drugs makes it challenging to interpret Vd without additional information.
Elimination half-life (t½)
It is the time taken for the concentration of a drug to decrease to half of its starting dose in the body after absorption is complete & distribution between body compartments has attained equilibrium.
- t½ can vary from a few hours to a few days or sometimes weeks.
- The absorption half-life should not be confused with the elimination half-life, as Typical absorption half-lives are less than 0.5 hours.
Maximum peak plasma concentration (Cmax)
It is the maximum concentration that a drug achieves in a specified compartment after it has been administered. (i.e., the point of max concentration of drug in plasma); measured in µg/ml or ng/ml.
- Cmax is a significant parameter because a drug with very low Cmax may be ineffective, while a high Cmax may lead to toxicity if the drug’s effect is known to be concentration-dependent.
- Knowing Cmax is crucial for adjusting drug dosages to ensure the intended therapeutic benefit while minimizing potential adverse effects.
Time of peak drug concentration (Tmax)
It is the time taken to reach the maximum drug concentration in plasma, measured in hours or minutes. It describes the time at which the absorption rate is maximum after drug administration.
- At Tmax rate of drug absorption is exactly equal to the rate of drug elimination.
- Small Tmax means rapid drug absorption.
Total plasma clearance (CL)
It is equal to the rate at which a certain drug is eliminated from plasma divided by the concentration of that drug in plasma. The overall ability of the body to clear a drug is the combined effort of the kidneys (renal clearance), the liver (hepatic clearance), and other tissues.
Clearance can be considered in two ways:
- When applied to the whole body, it represents the total elimination of the drug through various processes, including metabolism and excretion (an overall measure of how efficiently the body clears the drug).
- or examined at the organ level, which helps in understanding how individual organs (e.g., liver or kidney) contribute to drug elimination. This distinction is useful in pharmacokinetics for determining dosing regimens and assessing the impact of organ function on drug disposition.
Elimination rate constant (Kel)
It is a value that describes the rate at which a drug is removed from the system (i.e., is the fraction of a drug removed per unit time measured at any particular time).
The cumulative amount of drug excreted up to time t (Xu)
It is the sum of the amounts of drug excreted during that time t. By tracking the total amount of unchanged drug excreted in urine, valuable information can be obtained about how the body handles a drug.
- This method allows determining some key parameters like:
- The elimination rate constant
- The absorption rate constant
- The fraction and percentage of the drug absorbed
- The total amount absorbed
- The extent of bioavailability.
References
- Biopharmaceutics and Pharmacokinetics – V. Venkateswarlu, University College of Pharmaceutical Sciences, Kakatiya University, Warangal, Andhra Pradesh.
- ICH Guidelines – European Medicines Agency
👉Drug Repurposing: A valuable asset in the advancing of drug discovery process