1. Introduction: When Electrogenomic Formulations Begin to Read Genes
Only a molecular pulse, a sudden spike in mRNA transcripts firing like distress flares within the cytoplasm. Before the first symptom appears, before any clinical marker rises, the cell sends its earliest warning: A gene switches on, A survival pathway ignites, A disease signature takes its first breath.
Imagine stepping inside the body at the exact moment a cell senses the first hint of danger. There is no swelling or pain yet, no fever or fatigue, only a molecular pulse, a sudden rise in mRNA transcripts firing like silent distress flares within the cytoplasm. Before any symptom reaches the surface, before medicine even knows something is wrong, a gene flips its switch, survival pathways ignite, and disease begins its earliest whisper.
Now imagine! a Electrogenomic formulations so intelligent and exquisitely tuned to the biology of life that it responds instantly to that whisper, releasing treatment exactly when pathological gene expression crosses a danger threshold. Not guided by pH changes or temperature shifts, not controlled by human dosing schedules or guesswork, but triggered directly by the live genetic decisions happening inside the tissue itself.
What if medicine could respond to that moment?
Not when the disease is visible, but when it is just beginning to think.
Imagine! a formulation that doesn’t wait for inflammation to emerge. A therapy so attuned to the biology of the cell that it releases its drug payload the instant pathological gene expression crosses danger level, not an hour later, not a day later, but in real-time, in rhythm with the body’s own molecular language. No reliance on pH gradients. No dependency on temperature shifts. No guesswork around absorption kinetics. Just precise, intelligent communication, gene expression in, drug action out.
This is the birth of Electrogenomic Formulations, advanced drug systems that decode mRNA or microRNA patterns and respond only when disease attempts to take control.
This is the world of Electrogenomic Formulations, advanced drug systems that decode mRNA and microRNA signals and release therapy only when the body’s own molecular language calls for help. In this future, medicine is not something we administer to the body, but something that collaborates with it.
For generations, formulation scientists were sculptors, blending powders to create stable tablets and predictable release profiles. Then they became engineers, building nanoparticles and smart polymers that could navigate complex pathways. But now, they evolve into interpreters of biology, designing systems that understand transcripts, respond to cellular speech, and act in harmony with the rhythms of gene expression. Should seizure treatment begin after a seizure strikes, or before the brain even fully recognizes the event?
A New Kind of Intelligence in Formulation Science
To understand why this moment is so revolutionary, we need to rethink what gene expression actually represents. mRNA is not just a molecule floating aimlessly in a cell, it is a decision. It is the moment a cell chooses a path: to inflame or to heal, to divide or to die, to call for help or to surrender. Every transcript is a message, a declaration of intent long before symptoms ever reach the surface.
When inflammatory genes surge, the cell is saying: “I am under attack.” When oncogenes rise, it is whispering: “I am losing control.” When neuroinflammatory pathways flare, neurons call out: “Something is wrong inside my circuits.”
Now imagine! a formulation that can hear these messages while they are still whispers, long before disease shouts. A system that watches gene expression in real time and releases therapy only when the cell crosses an invisible threshold of danger. A material that doesn’t just sit in the body waiting to dissolve, but listens, interprets, and acts with precision.
And once we unlock that level of partnership between medicine and the genome, the line between therapy and biology will blur forever.
2. The Scientific Spark Behind the Revolution
Where biology begins to speak, and materials finally listen.
Breakthroughs rarely arrive fully formed. They begin as unrelated experiments scattered across disciplines. But occasionally, the world shifts when those separate sparks connect. The rise of electrogenomic formulations is one of those moments, a fusion of technologies that were never meant to meet, yet now create a language medicine can finally understand. The origins of electrogenomic formulations come from unexpected places:
2.1 RNA-Responsive Hydrogels

At ETH Zürich and Seoul National University (2023–2024), researchers engineered hydrogels that move, literally, when they detect specific microRNA sequences.
How? They embedded hairpin DNA strands into the gel. When a target miRNA (elevated in cancer or inflammation) binds to the hairpin, the gel swells or contracts, as if reacting emotionally to genetic distress.
Suddenly, material behavior becomes a direct consequence of gene expression. Not pH. Not temperature. Not time. But the cell’s voice.
2.2 CRISPR-Diagnostic Microchips
Systems like SHERLOCK and DETECTR (2022) were built for disease diagnostics, detecting mRNA at single-molecule sensitivity.
But a visionary idea emerged:
What if the moment CRISPR detects a pathological mRNA signal, it immediately deploys therapy? Detection becomes action. Monitoring becomes healing.
Imagine!
- A spike in inflammatory mRNA → anti-inflammatory release.
- An oncogene activation → local chemotherapy pulse.
- A stress-circuit flare → neuroprotective micro-dose
CRISPR isn’t just a gene editor anymore. It’s a molecular stethoscope with a trigger.
2.3 Electrically Amplified Gene Sensing
MIT scientists (2023) built bioelectronic transistors that convert gene expression into electrical signals.
For the first time: Gene activation → creates a readable digital pulse
It means drug systems can measure genetic distress the same way a smartwatch measures your heart rate.
This is the crossover point where:
- Formulation science meets electronics.
- Genomics becomes data.
- Drug release becomes algorithmic.
The body becomes the interface.
2.4 Synthetic Promoters linked to nanoparticle release (2024)
Harvard (2024) engineered nanoparticles coated with synthetic promoters, tiny DNA switches tuned to specific genes like NF-κB, the master regulator of inflammation.
When NF-κB rises: The promoter flips on → the nanoparticle membrane bursts → drug is freed
Not because time passed. Not because a coating dissolved. Because the gene itself asked for help. This is medicine that honors the cell’s timing, not ours. For the first time, a formulation could be “programmed” to release medication only when genes misbehave.
3. The Human Moment: Why It Matters More Than Anything Else
Picture a patient living with rheumatoid arthritis!
This molecular shift happens hours before a flare, yet traditional medicine is blind to it. The tablets and injectables we prescribe operate on rigid timing, not dynamic biology. They do not know what the cell knows.
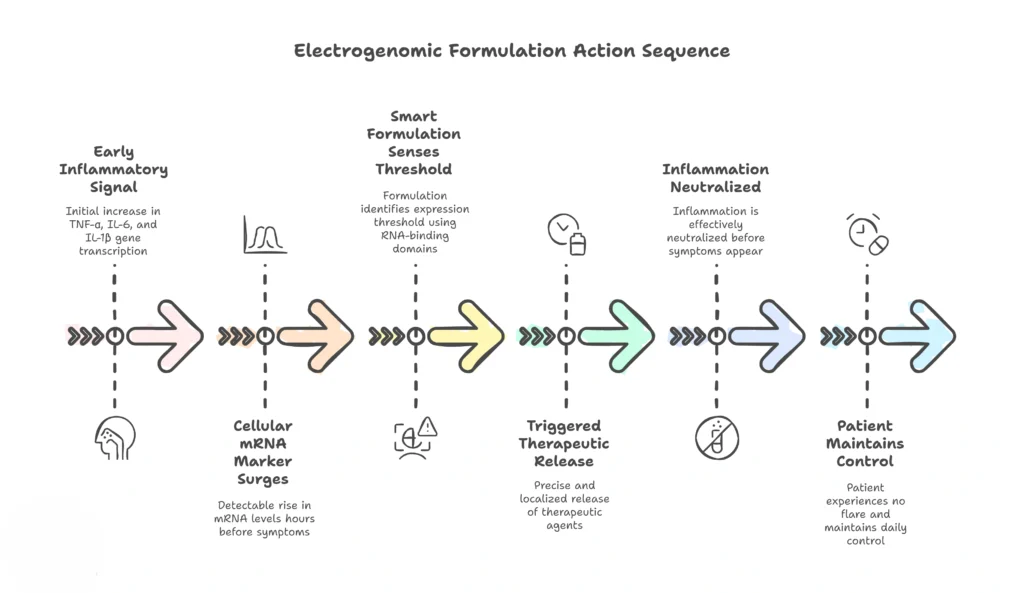
Now imagine another scenario, a future only inches away from our present. Within that same joint resides a gene-sensing formulation: a targeted depot or nanoparticle armed with an API and intelligent material coding. As inflammatory gene expression rises, these nanodevices recognize a diagnostic signature, an mRNA fingerprint of pathology.
Electrogenomic formulations responds, It releases a micro-dose of therapy, just enough to neutralize the fire before it spreads. This is more than personalized medicine. This is participatory medicine, where the electrogenomic formulations becomes a guardian that stands vigil inside the body.
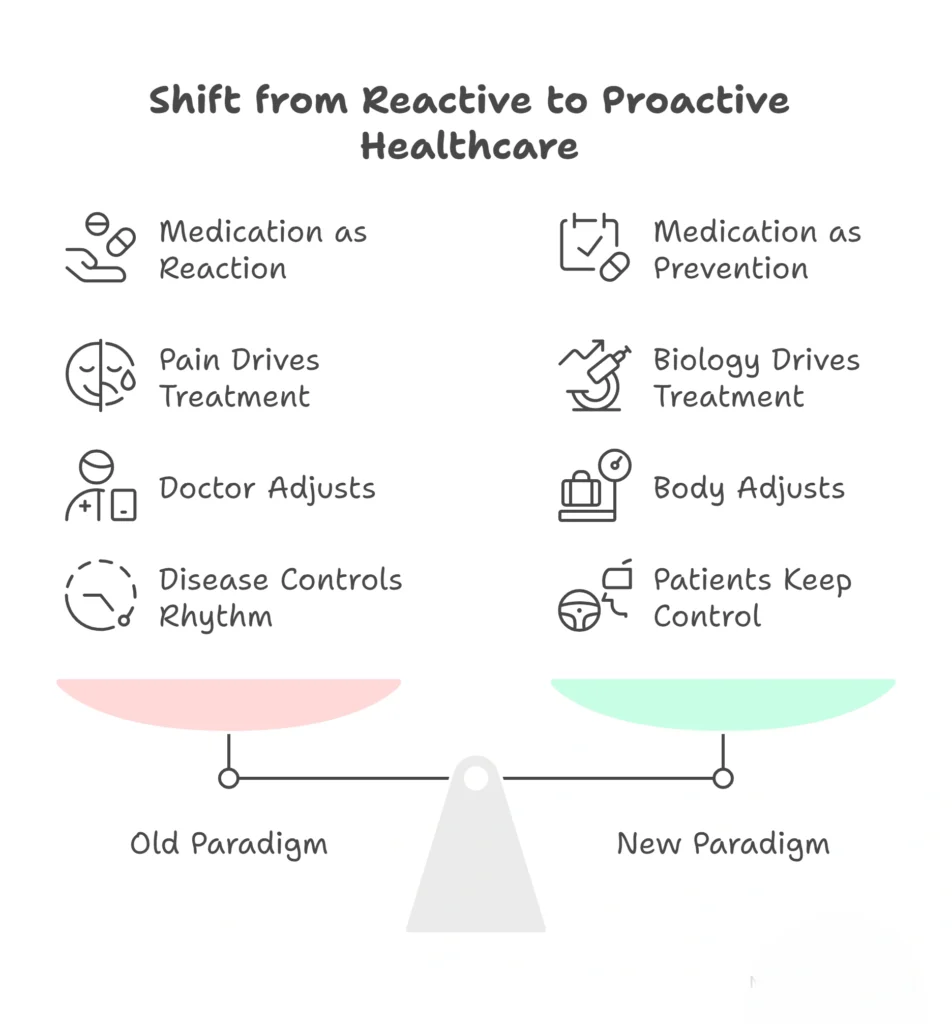
It is a shift from treating symptoms to anticipating biology. From fixing disease to intercepting it. From uncertainty to peace of mind. For patients who wake every day expecting pain, a therapy that acts before they suffer is not technology, It is dignity.
Flowchart: How Electrogenomic Formulations Prevents a Flare Before It Starts
4. The Emotional Weight of the Scientific Leap
There is a moment in science when technology stops feeling mechanical and begins to feel alive. Electrogenomic formulations sit exactly at that moment.
For the first time in medical history, the patient’s own cells become the dosing authority. Not a timer. Not a physician’s estimate. Not a fixed-release polymer struggling to guess the right moment.
Here, biology writes the prescription in real time, Instead of medicine being imposed on the body, we see a partnership emerging:
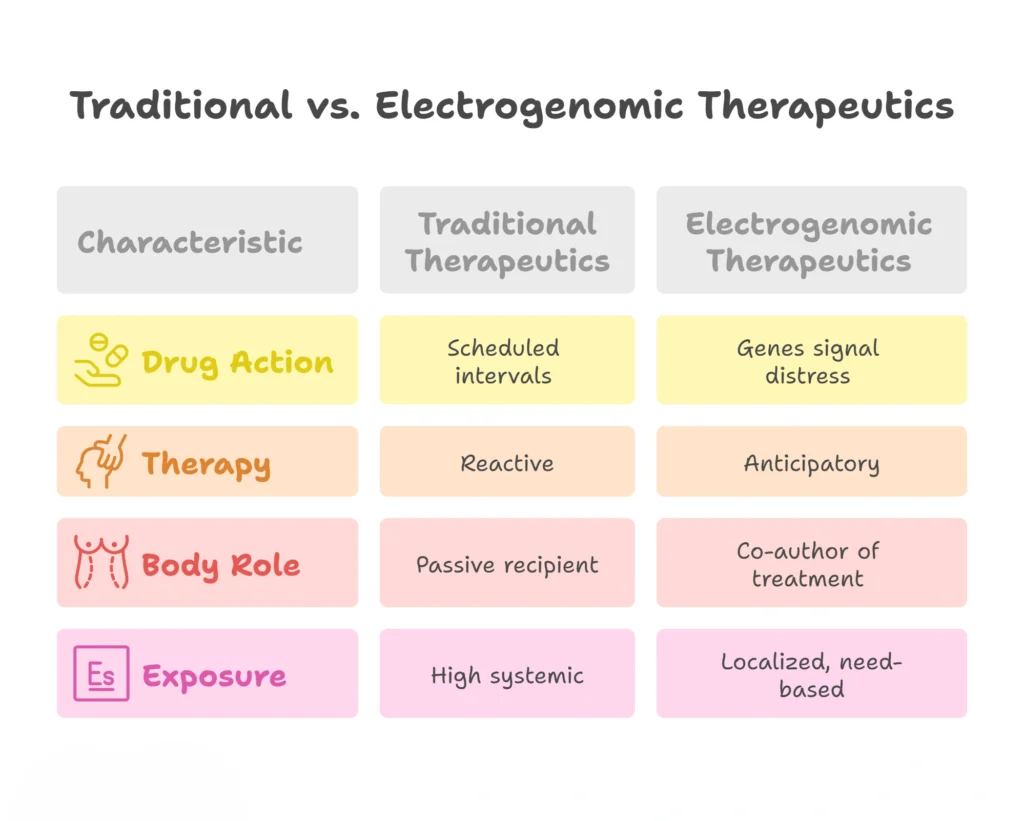
This shift seems small on paper, but philosophically, it changes everything. It transforms disease control from a battle into a conversation.
Imagine!
A child living with epilepsy: unaware that their neurons have just begun expressing early seizure-related mRNA. Before any physical tremor arises, nanoparticles stationed near vulnerable neural circuits sense the molecular deviation. They respond instantly, releasing micro-doses of anticonvulsant inside the very networks preparing to misfire. No panic.
No ambulance. Just calm continuity, because the therapy listened.
A patient undergoing chemotherapy: their tumor cells quietly increasing expression of KRAS or MYC oncogenes. Before metastasis gains momentum, a genetic “key” unlocks the membrane of a nano-depot embedded inside the tumor bed. The cytotoxic agent is released not broadly, but locally, precisely where malignant instructions were spoken. Medicine doesn’t just kill cancer, it interrupts the conversation that fuels it.
Someone with rheumatoid arthritis: once tormented by unpredictable flares, walks through their day without fear. As soon as synovial cells upregulate inflammatory transcripts, a smart electrogenomic formulations detects the deviation and delivers a micro-pulse of immunomodulation before a single joint swells. The patient never even knows a battle was prevented.
5. The Physics, Chemistry, and Biology of Electrogenomic Triggers
The beauty of electrogenomic formulations drug delivery lies in how effortlessly it stitches together three worlds that have historically lived apart: biology, where gene expression is decided; chemistry, where responsive materials interpret those decisions; and physics, where molecular forces transduce those biological whispers into therapeutic actions.
At first glance, the idea sounds almost mystical, am electrogenomic formulations that “reads” gene expression and reacts. But when you zoom in to the molecular scale, the mechanism becomes not only clear, but astonishingly elegant.

Fusion of Physics, Chemistry & Biology: A glowing strand of mRNA floats at the center as intelligent polymers shift around it, responding to its signal. Soft electric currents ripple through the scene, revealing the physics at work, while the silver-white outline of a cell frames the biology beneath. In one moment, chemistry, physics, and biology merge into a single system learning to read a gene.
How does a Electrogenomic formulations “read” gene expression?
It begins at the level of the cell’s own voice: mRNA. When a gene becomes active, it releases a surge of mRNA transcripts into the cytoplasm, tiny strands of biological intent. These are not random molecules; they are the most honest indicators of what a cell is preparing to do next. Cancer cells express oncogenes before a tumor grows. Inflamed tissues upregulate cytokine transcripts before symptoms appear. Neurons release distinct mRNA patterns before misfiring into seizure.
Electrogenomic formulations take advantage of this moment, the molecular decision point, long before disease manifests.
The Biology: Gene Expression as a Signal
Every gene produces a unique RNA “signature.” These signatures are as recognizable as fingerprints. A formulation designed for rheumatoid arthritis, for example, may be tuned to detect surges in TNF-α or IL-1β transcripts, while one designed for epilepsy may respond to rising c-Fos or Arc expression in hyperactive neurons.
Inside the formulation, RNA-responsive elements, such as hairpin DNA structures or CRISPR-based sensors, bind only to the target mRNA. When this binding occurs, the biological signal, once abstract, becomes a physical change within the material.
The Chemistry: Translating RNA Binding into Material Motion
This is where chemistry takes the baton. In many electrogenomic systems, the core is a hydrogel or nanoparticle engineered with RNA-sensitive lock-and-key domains. When the correct mRNA binds, it doesn’t just sit there, it changes the chemistry of the material. DNA hairpins unfold. Polymer chains swell. Crosslinks break. Hydrophobic pockets collapse.
These tiny conformational changes accumulate, transforming the material’s structure in a way that either opens pathways for drug diffusion or triggers the rupture of nanoscale reservoirs. It is chemistry behaving like a sensor, a switch, and a gatekeeper, all at once.
The Physics: Converting Tiny Genetic Signals into Therapeutic Movement
And then comes the physics: how does a nanometer-scale chemical shift turn into a macroscale therapeutic effect? Through molecular transduction, the same principle that underlies every responsive material in nature.
Chemical deformation creates internal tension. Tension becomes mechanical expansion. Expansion becomes pore formation. And pores become the gateway through which the API escapes into living tissue.
Some systems amplify this effect electrically. Imagine a nanoparticle that not only binds RNA but also carries an embedded bioelectronic element. When mRNA binds, the local charge changes, and a conductive polymer shifts its electron distribution. That shift alters the polymer’s conformation, accelerating drug release.
Physics, chemistry, and biology merge so smoothly that it becomes hard to tell where one ends and the other begins.
Why This Matters?
This means a formulation doesn’t “wait” for inflammation or seizures or relapse, it responds to the gene expression changes that precede those events. Drug delivery becomes preventive rather than reactive. Unlike traditional systems based on clocks, coatings, or pH triggers, electrogenomic formulations synchronize themselves with the earliest biological signal available, effectively becoming the first line of defense.
For patients, this could mean the difference between a flare avoided and a flare endured. For formulators, it marks the beginning of an entirely new scientific language.
Conclusion: Where Medicine Learns to Anticipate Life
For centuries, medicine has chased disease from behind, waiting for swelling to show, waiting for neurons to misfire, waiting for tumors to announce their presence. We have always reacted, and often too late. Electrogenomic formulations rewrite that story. They respond to the very first shift inside a cell, the moment a gene begins to drift toward danger. No symptoms. No guessing.
Just biology signaling for help, and therapy answering immediately. This isn’t simply precision medicine; it is the first era of pre-symptomatic protection, where the body itself becomes the trigger for healing. A child no longer fears the next seizure because their medication prevents the storm before it forms. A person living with autoimmune disease wakes up without dread because their therapy responds the instant inflammation stirs beneath the surface.
If neuroresponsive formulations gave medicine the ability to hear the electrical language of the brain, electrogenomic formulations system now let it understand the genetic language of the cell itself.
A cancer survivor breathes easier knowing relapse cannot silently grow. What began with neuroresponsive drug formulations sensing the brain’s electrical whispers now evolves further, into systems that listen to the genome’s earliest warnings. This is medicine that does not just act on the body, but acts with the body. A guardian embedded in tissue. A partner that protects while disease is still only a thought. And if this is what the beginning looks like, imagine what comes next.
References
- Polymeric nanocarriers with stimuli-responsive properties for drug delivery. GSC Biological and Pharmaceutical Sciences (2022).
- Zhang, Y. & Wu, B. M. Current Advances in Stimuli-Responsive Hydrogels as Smart Drug Delivery Carriers. Gels (2023).
- Multifunctional and stimuli-responsive nanocarriers for targeted therapeutic delivery. PubMed (2025).
- Wang, J. et al. Developing mRNA Nanomedicines with Advanced Targeting Functions. Nano-Micro Letters (2025).
- Nanostructures enabling stimuli-responsive drug delivery and biosensing. RSC Chemical Biology (2025).
- Precise intracellular uptake and endosomal release of mRNA via nanogels. PubMed (2025).