
Introduction:
In today’s rapidly evolving global environment, organizations face unprecedented volatility, uncertainty, complexity, and ambiguity. This reality has been magnified in sectors like pharmaceuticals, where regulatory changes, technological advancements, supply chain vulnerabilities, and shifting patient demands converge. In such an environment, the traditional command-and-control organizational structures often fail to deliver both speed and compliance. To survive and thrive, organizations must develop resilience, and at the core of resilience lie two interdependent capabilities: agility and flexibility.
Agility refers to the organization’s ability to sense change, make rapid decisions, and act decisively, while flexibility denotes the capacity to adapt structures, processes, and resources in response to evolving circumstances. Together, they enable organizations to navigate disruptions efficiently, maintain high-quality standards, and innovate continuously without compromising compliance or operational stability. This article provides a comprehensive exploration of agility and flexibility, with particular emphasis on pharmaceutical operations, offering insights into conceptual frameworks, organizational capabilities, leadership behaviors, cultural enablers, and practical implementation strategies.
Understanding The Modern Organizational Environment:
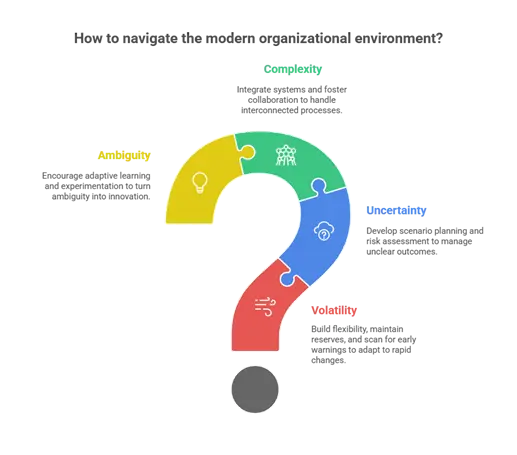
The modern organizational environment is increasingly defined by four interrelated factors:
- Volatility:
Volatility in the pharmaceutical industry refers to rapid and unpredictable changes that can disrupt operations and strategy. This could include sudden fluctuations in raw material availability, shifts in healthcare policies, or technological breakthroughs that render existing processes or therapies less competitive. For example, a sudden shortage of an active pharmaceutical ingredient (API) due to geopolitical tensions or natural disasters can halt production lines, delay drug launches, and strain supply chains. Organizations must therefore build flexibility into their operations, maintain strategic reserves, and continuously scan the environment for early warning signals to adapt swiftly to such dynamic conditions. - Uncertainty:
Uncertainty arises when organizations face situations with unclear outcomes, such as evolving regulatory guidelines, competitor strategies, or changing patient needs. In pharmaceuticals, this might include ambiguous clinical trial results, unexpected side effects, or uncertain market acceptance for a new therapy. Leaders often have to make decisions with incomplete information, balancing risk and opportunity while ensuring patient safety and regulatory compliance. Developing scenario planning, risk assessment frameworks, and agile decision-making processes helps organizations navigate this fog of uncertainty effectively. - Complexity:
Complexity in modern pharmaceutical organizations arises from the complicated interconnected processes, multi-site operations, and numerous stakeholders. Drug development involves research, clinical trials, manufacturing, regulatory compliance, and marketing, all of which must coordinate seamlessly across different teams and geographies. A delay or error in one function can propagate through the organization, affecting product quality, launch timelines, and financial performance. Effective management of complexity requires integrated information systems, cross-functional collaboration, and strong communication channels that allow organizations to anticipate interdependencies and respond proactively. - Ambiguity:
Ambiguity occurs when information is incomplete, contradictory, or rapidly changing, making decision-making particularly challenging. For example, during a pilot batch, unexpected dosage form behavior may create unclear interpretations, leaving researchers and executives uncertain about the next steps. Similarly, shifts in healthcare policy or competitive intelligence can provide conflicting guidance for strategic planning. To navigate ambiguity, organizations must foster a culture of adaptive learning, encourage experimentation, and support leaders who can make timely decisions despite incomplete data, turning ambiguity into an opportunity for innovation rather than a barrier.
Pharmaceutical organizations operate within an intensely regulated environment, which adds a layer of structural rigidity that can clash with the need for speed. Volatility in supply chains, uncertainty in regulatory approvals, complexity in global manufacturing networks, and ambiguity in emerging therapeutic areas all challenge traditional operational models.
What is meant by A Resilient Organization?
A resilient organization possesses the capacity to anticipate, prepare for, respond to, and recover from internal and external disruptions while maintaining its core functions, purpose, and strategic direction. In the pharmaceutical industry, resilience goes beyond simply weathering challenges such as supply chain disruptions, regulatory changes, or new product trials setbacks; it encompasses the ability to adapt processes, innovate under pressure, and sustain organizational learning in the face of uncertainty and change. The foundation of resilience rests upon agility and flexibility while embedding robust problem-solving, risk management, contingency planning, and knowledge-sharing practices across all levels. Agility enables rapid response and decision-making. Flexibility provides structural capacity to reallocate resources, reorganize processes, and adjust strategies without compromising compliance or performance.
Defining Agility and Flexibility:
The terms agility and flexibility are often used interchangeably in organizational discussions, yet they represent two distinct approaches to managing change, making decisions, and shaping strategy. Both are essential for high-performing teams, especially in dynamic and highly regulated industries like pharmaceuticals, but they operate in complementary ways.
Flexibility is fundamentally a reactive capability. It reflects an organization’s ability to adjust, adapt, or bend in response to changing circumstances without losing integrity. In the pharmaceutical context, flexibility may involve reassigning research staff to urgent projects, modifying clinical trial protocols in response to unexpected patient outcomes, or shifting production schedules to address raw material shortages. Flexible organizations can absorb shocks, maintain continuity, and ensure that operations continue smoothly despite unplanned disruptions.
Agility, in contrast, is proactive and forward-looking. It involves anticipating change, responding swiftly, and pivoting strategically with purpose. Agile pharmaceutical teams do more than react; they identify emerging opportunities or risks and adjust their strategies before external pressures fully manifest. For example, an agile research and development team may accelerate a promising drug candidate in response to an emerging public health threat, or a commercial team may launch targeted campaigns ahead of a competitor’s product entry. Agility is about speed, foresight, and strategic responsiveness, turning change into an opportunity for innovation, learning, and growth rather than merely a challenge to overcome.
While distinct, agility and flexibility are deeply interdependent. Flexibility provides the structural and operational foundation that enables agile actions. Without flexible processes, adaptive resources, and cross-trained personnel, proactive initiatives may falter or create unnecessary risk. Conversely, agility gives flexibility, direction, and purpose, ensuring that reactive adaptations are aligned with strategic objectives and delivered with impact. In the pharmaceutical industry, organizations that cultivate both capabilities can respond to uncertainty with confidence, pivot when opportunities arise, and maintain resilience in the face of disruption.
Organizational Examples
- Agility without flexibility: Quick decision-making is hampered if processes cannot adjust to new needs.
- Flexibility without agility: Resources can adapt, but slow decision-making delays execution.
- High agility and high flexibility: Rapid, coordinated, and adaptive responses that maintain quality and compliance.
In short, flexibility is reactive, allowing organizations to adapt to change, while agility is proactive and dynamic, enabling them to anticipate, respond, and evolve strategically. Together, they form a powerful dual capability essential for thriving in today’s volatile, uncertain, complex, and ambiguous business environment.
Agility and Flexibility Frameworks in Modern Organizations:
In essence, agility frameworks provide structured, proactive approaches to anticipate and strategically act on change, while flexibility frameworks enable reactive adjustments that allow organizations to absorb shocks and adapt efficiently. Together, they form a complementary system: flexibility provides the adaptability that allows agile initiatives to succeed, and agile frameworks give strategic direction and speed to the reactive capabilities of flexibility. This combination is particularly critical in the pharmaceutical sector, where balancing innovation, regulatory compliance, operational efficiency, and patient safety is essential for sustainable success.
Agile Frameworks
1. Scrum: Scrum is an iterative and incremental framework designed to manage complex projects, most commonly in software development but increasingly in pharmaceutical R&D and project management. Work is divided into short cycles called sprints, enabling teams to plan, execute, and review work continuously, adjust priorities, and respond rapidly to emerging challenges such as unexpected clinical trial results or regulatory feedback.
2. Kanban: Kanban is a visual workflow management system that limits work-in-progress and focuses on continuous flow. In pharmaceutical operations, Kanban can be applied to laboratory work, production scheduling, or regulatory submissions to track tasks, identify bottlenecks, and ensure smooth, predictable progress while maintaining quality standards.
3. Disciplined Agile: It provides a toolkit to help organizations select the most suitable agile practices based on context, goals, and scale. Pharmaceutical companies can use DA to balance innovation, risk management, and regulatory compliance, tailoring agile approaches to project complexity, team size, or therapeutic area.
4. Scaled Agile Framework (SAFe): SAFe is a set of principles and practices for scaling agile methods to large enterprises, designed to align and synchronize multiple agile teams to work on common goals. In pharmaceutical organizations, SAFe can align cross-functional teams across R&D, quality, regulatory affairs, and manufacturing, ensuring that strategic objectives are consistently executed while enabling rapid response to scientific discoveries or market opportunities.
SAFe core values:
1. Alignment: Strategies and tactics are synchronized across all levels of the organization to achieve business agility and deliver value.
2. Built-in Quality: Quality practices are embedded throughout every process rather than relying solely on end-stage inspection.
3. Transparency: The organization operates in an environment of trust. All data is visible, problems are exposed, and decisions are made based on reliable information.
4. Program Execution: Working solutions and business values meet customer needs and are delivered in the shortest sustainable lead time.
For more information on the Scaled Agile Framework, see the video below:
Flexibility Frameworks
- Flexibility Framework: This framework helps organizations identify the types of flexibility they require, such as the ability to modify processes, reallocate resources, or adjust priorities with minimal cost in time, effort, or performance. In pharma, this might involve reallocating staff between trials or modifying supply chain routes in response to sudden raw material shortages.
- Business Process Flexibility: This approach focuses on designing processes that can adapt quickly to external and internal changes without significant penalties. Examples in pharmaceuticals include adaptive clinical trial protocols, modular manufacturing processes, and scalable quality management systems that can accommodate new regulatory requirements.
- Organizational Flexibility: This encompasses the ability to adjust organizational structures, workflows, and even business models to meet evolving market and customer needs. For pharmaceutical companies, organizational flexibility might involve creating cross-functional teams for emerging therapeutic areas, decentralizing decision-making for faster responsiveness, or pivoting commercial strategies in response to competitor actions or patient demand.
How to measure Agility and Flexibility of an Organization?
Agility KPIs measure an organization’s proactive ability to sense, respond, and capitalize on change quickly and strategically. These indicators track how effectively teams anticipate challenges, make decisions, and execute initiatives in a rapidly evolving environment. Examples of agility KPIs in the pharmaceutical industry include:
- Time-to-Decision: Average time taken for teams to make critical project or operational decisions, e.g., approving protocol changes in technology transfer.
- Time-to-Market: Duration from concept to product launch, reflecting the speed of product development and commercialization.
- Change Response Rate: Speed at which the organization adapts processes or resources in response to external or internal changes, such as regulatory updates or market demand shifts.
- Innovation Velocity: Number of new product formulations, process improvements, or initiatives successfully executed within a defined period.
- Cross-Functional Collaboration Index: Measurement of coordination effectiveness between teams (e.g., R&D, regulatory, quality and manufacturing) to enable rapid response.
Flexibility KPIs measure an organization’s reactive capacity to adjust structures, resources, and processes efficiently without major disruption. These indicators evaluate how well the organization absorbs shocks, reallocates resources, and adapts to changing conditions. Examples of flexibility KPIs in pharmaceuticals include:
- Resource Reallocation Time: Time required to reassign staff, equipment, or materials to priority projects or new demands.
- Process Adaptation Speed: Time taken to modify workflows, manufacturing lines, or trial protocols in response to operational or regulatory changes.
- Operational Downtime: Duration of interruptions due to adjustments or changes, reflecting efficiency in adaptation.
- Scope Change Accommodation: Number of project or process modifications successfully incorporated without compromising quality or compliance.
- Organizational Resilience Index: Assessment of how well the organization maintains core functions and performance when facing disruptions or unexpected events.
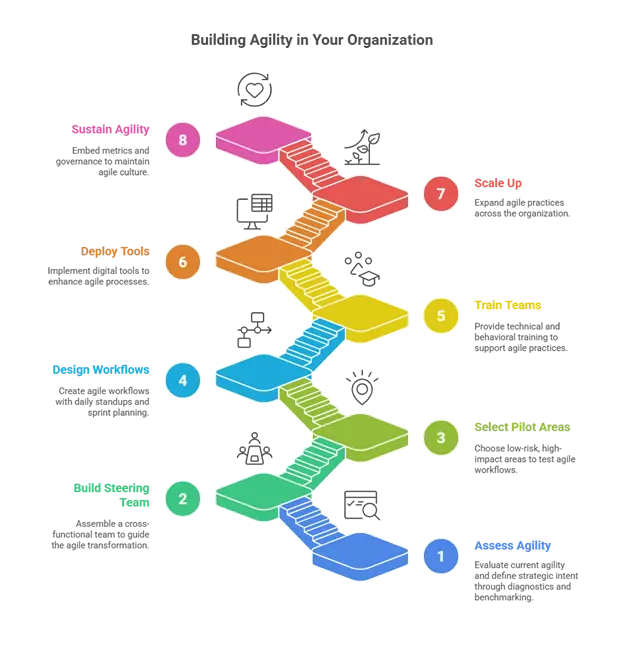
How to build Agility in your organization?
- Assess Current Agility and Define Strategic Intent: Diagnostics, benchmarking, value stream mapping.
- Build Cross-Functional Steering Team: Includes QA, QC, Production, Supply Chain, Regulatory, R&D, IT, People Operations, Finance.
- Select Pilot Areas: Low-risk, high-impact areas like change control or low profile markets.
- Design Agile Workflows: Daily standups, sprint planning, sprint reviews, retrospectives, visual management systems.
- Train Teams: Technical (Lean, RCA, risk-based thinking, digital tools) and behavioral (collaboration, communication, adaptive leadership).
- Deploy Digital Tools: MES, LIMS, ERP, eQMS, predictive analytics dashboards.
- Scale Up: Standardize agile SOPs, expand across value streams, share lessons learned.
- Sustain Agility: Embed metrics, incentives, and governance to reinforce culture and continuous improvement.
The Role of Leadership and Organizational Culture:
Leadership and organizational culture play a decisive and transformative role in enabling true agility and flexibility within modern organizations especially in highly regulated, fast-evolving industries like pharmaceuticals. Agility and flexibility do not emerge from tools, frameworks, or processes alone; they are ultimately shaped by how leaders think, behave, and communicate, and by the collective norms, values, and beliefs that guide daily actions across the organization. When leadership and culture align to support proactive thinking, rapid adaptation, and openness to change, organizations become more resilient and capable of navigating the complex business environment.
The Role of Leadership
Leaders are the catalysts of agility. Their behaviors set the tone for how teams respond to challenges, pursue innovation, and handle uncertainty. Agile leadership is fundamentally different from traditional command-and-control styles. Rather than directing, prescribing, or micromanaging, agile leaders empower their teams, share decision-making authority, and create an environment where experimentation is encouraged and swift adjustments are possible. This requires humility, trust, and a willingness to relinquish hierarchical control. Leaders must communicate clear strategic priorities while allowing teams the autonomy to determine how best to achieve them. Effective leaders also remove obstacles, whether bureaucratic, structural, or resource-related so teams can operate at speed. They foster psychological safety, encourage teams to speak up, identify risks early, and challenge outdated practices without fear of blame. In short, leaders create the conditions that make agility and flexibility not only possible but sustainable.
The Role of Organizational Culture
Culture is the soil in which agility grows or fails to thrive. Even with the most advanced frameworks, an organization with a rigid, risk-averse, or siloed culture will struggle to be agile. A culture that supports agility is rooted in openness, collaboration, continuous learning, and adaptability. Employees feel comfortable sharing ideas, proposing changes, and experimenting with new approaches. Mistakes are viewed as learning opportunities rather than grounds for punishment. Such a culture encourages a bias toward action and short feedback loops, helping organizations respond quickly to emerging issues and shifting demands. Flexibility also depends heavily on cultural norms, specifically, a willingness to adjust roles, processes, and priorities without resistance or territorial behavior. Cultures that value transparency and knowledge sharing will naturally adapt more efficiently than those that cling to silos and rigid procedures.
Leadership/Culture Interdependence
Leadership and culture are inseparable in implementing agility and flexibility. Leaders shape culture through daily actions, incentives, language, and priorities, while the culture reinforces or resists leadership efforts. If leaders champion agility but the culture punishes failure or discourages open discussion, agility initiatives will fail. Conversely, when leaders model agility by being transparent, adaptable, supportive, and collaborative, the culture gradually shifts to reflect these values. Over time, the organization becomes more responsive, proactive, and resilient. This alignment allows agility and flexibility to evolve from isolated initiatives into an organizational capability embedded in the company’s DNA.
Conclusion:
Agility and flexibility are strategic imperatives in building any resilient organization in the modern business environment. In pharmaceutical organizations, they enable rapid, coordinated decision-making while ensuring compliance and patient safety. Leadership behaviors, cultural norms, cross-functional collaboration, digital enablement, and iterative learning collectively enable these capabilities. Implementing structured roadmaps and nurturing an agile culture ensures organizations not only survive disruptions but thrive, achieving operational excellence and sustained competitiveness.
References:
- Denning, S. (2018). The Age of Agile: How Smart Companies Are Transforming the Way Work Gets Done.
- McKinsey & Company (2017). The 5 trademarks of Agile organizations.


Pingback: Navigating the Perfect Storm of Industry Challenges - Pharma Job AID