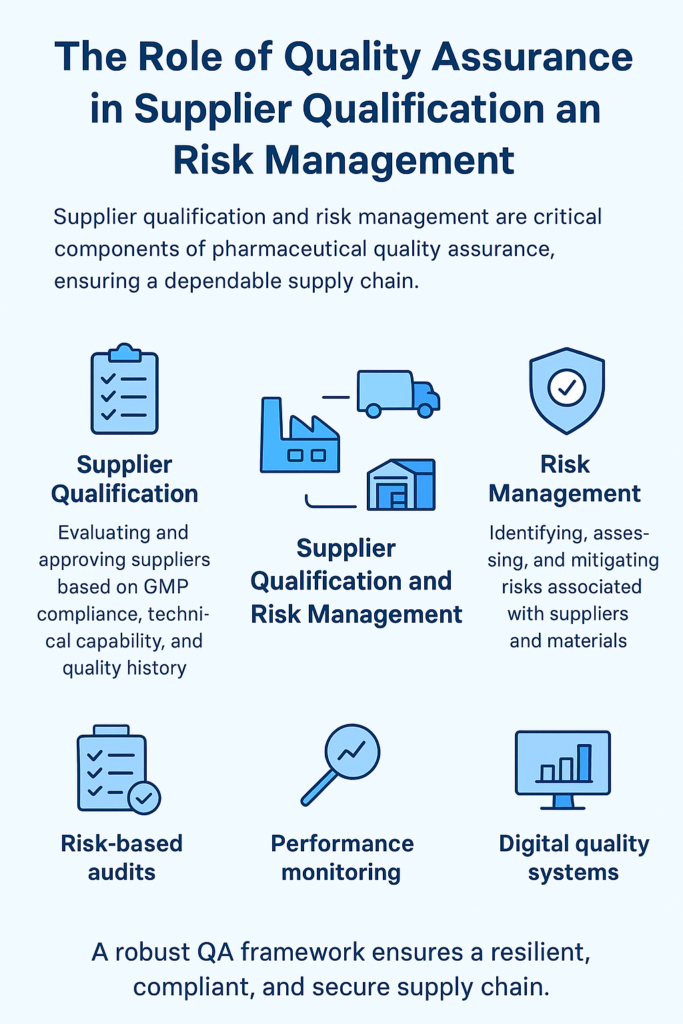
The modern Pharmaceutical landscape has expanded the scope of Quality Assurance beyond production, making it a critical function throughout the supply chain. Supplier qualification and risk management play a pivotal role in safeguarding product integrity, regulatory compliance, and patient safety.
This article examines how the expanded Quality Assurance (QA) functions encompass the evaluation, approval, and continuous monitoring of suppliers. It highlights systematic approaches to assess supplier capabilities, including auditing, performance tracking, and risk-based assessments. Emphasis is placed on identifying potential vulnerabilities in the supply chain, mitigating risks associated with raw materials, excipients, and packaging components, and establishing preventive strategies to maintain product quality.
By integrating supplier qualification with proactive risk management, pharmaceutical companies can enhance traceability, strengthen regulatory adherence, and reduce the likelihood of product recalls or patient harm. The insights presented serve as a practical guide for QA professionals to implement robust supplier oversight programs that align with Good Manufacturing Practices (GMP) and industry standards, ultimately reinforcing a culture of quality throughout the supply chain.
1. Introduction:
What is Supplier Qualification?
Supplier qualification is a systematic evaluation and approval process that determines whether a supplier can consistently meet quality, regulatory, and business requirements before providing materials or services.
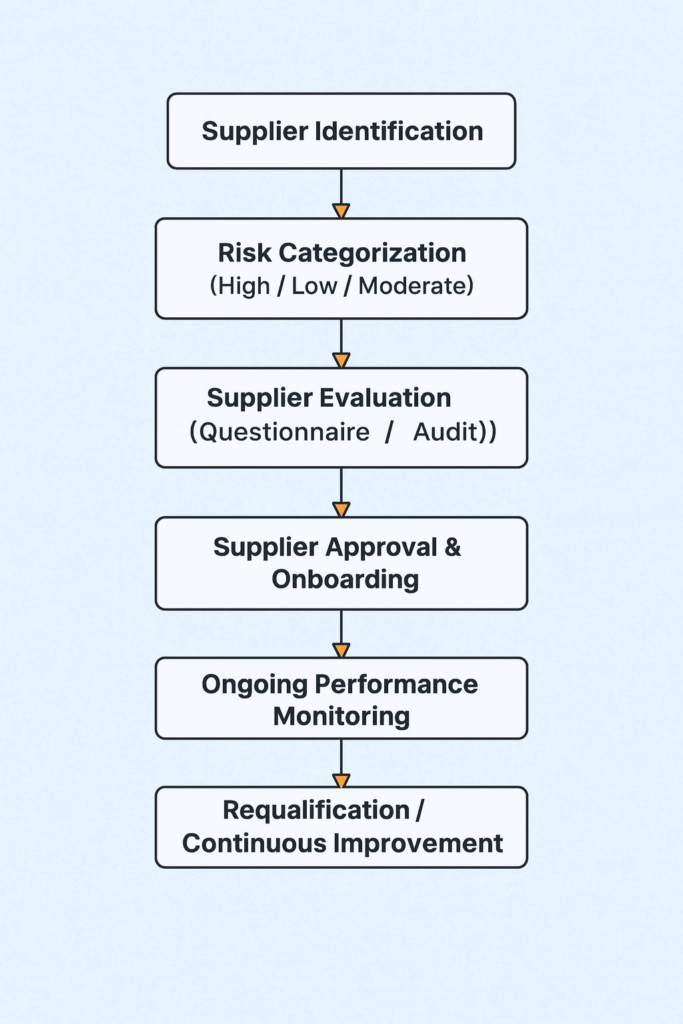
It is not a one-time activity it’s a life cycle approach that begins with supplier identification and continues through regular monitoring, performance evaluation, and re-qualification.
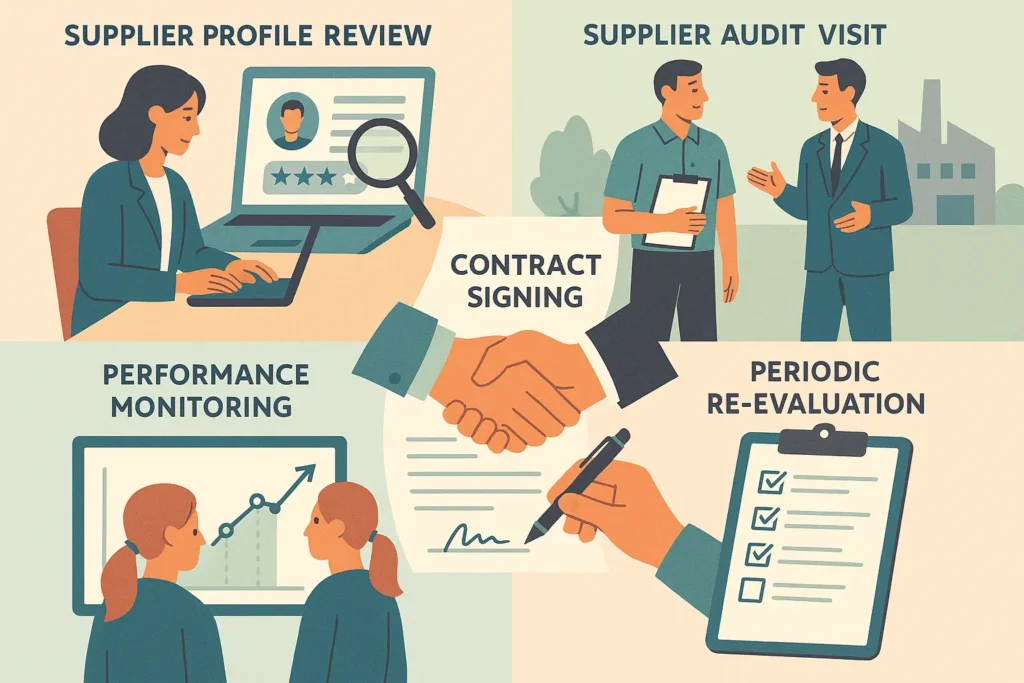
6 Steps in Supplier Qualification
1. Initial Screening
- Review supplier profile, certifications (ISO, GMP, GDP), and compliance history.
2. Risk Categorization
- Classify suppliers as critical, major, or non-critical based on material use and potential impact on product quality.
3. Detailed Assessment
- Conduct on-site or remote audits to evaluate the quality management system, documentation, and controls.
4. Technical/Quality Agreement (QTA)
- Establish mutual understanding of responsibilities, expectations, and quality metrics.
5. Ongoing Monitoring
- Track deviations, complaints, and CAPA closure rates to maintain consistent quality.
6. Re-qualification / Periodic Re-Evaluation
- Reassess suppliers at defined intervals or after significant changes. This ensures continued compliance, risk management, and alignment with GMP standards.
QA’s Key Responsibilities
- Define qualification criteria, including GMP standards, regulatory expectations, and internal SOPs.
- Review supplier questionnaires and supporting compliance evidence.
- Conduct or evaluate audit reports to confirm GMP adherence.
- Ensure QTAs clearly define responsibilities, quality metrics, and change control processes.
- Continuously monitor supplier performance to detect trends and address issues proactively.
A well-defined qualification system builds confidence in material quality, reduces supply risk, and ensures regulatory readiness at all times.
2. Regulatory Expectations:
Global regulatory agencies such as FDA, EMA, MHRA and WHO expect pharmaceutical companies to have documented, risk-based supplier management systems.
Key Regulatory Requirements
- Documented evidence of initial qualification and periodic re-qualification.
- Risk-based re-qualification, aligned with supplier performance and material criticality.
- Active Quality Agreements that address change control, deviations, complaints, and responsibilities.
- Apply Quality Risk Management (QRM) principlesas per ICH Q9 guidelines.
- Assurance of traceability and data integrity across the supply chain.
QA teams must remain inspection-ready at all times, with accessible audit reports, risk assessments, and CAPA documentation. Compliance is no longer optional; it is a critical factor in business continuity and regulatory approval.
3. Integrating Risk Management: (ICH Q9 & Q10)
Risk management is the cornerstone of modern QA. It allows teams to prioritize resources and focus efforts on suppliers or materials that pose higher potential risks.
Applying Risk Management in Practice
QA applies a structured risk management approach, often guided by ICH Q9 (Quality Risk Management) and ICH Q10 (Pharmaceutical Quality System):
| Step | QA Activity | Example |
| Risk Identification | Gather supplier data, compliance record, and market history | API supplier with previous warning letter |
| Risk Assessment | Evaluate severity × probability × detectability (FMEA) | High-risk rating for sterility-impacting materials |
| Risk Control | Implement audits, extra testing, or approval conditions | Quarterly audits for critical material suppliers |
| Risk Review | Trend analysis and periodic reassessment | Review of complaint data every 6 months |
Another example is that a supplier of sterile water bags was categorized as high risk after audit findings revealed inadequate environmental monitoring. QA increased audit frequency to semi-annual and implemented supplier CAPA verification. Within one year, deviations dropped by 90%, demonstrating how data-driven risk control strengthens supplier reliability.
This risk-based approach ensures that QA resources are focused where they are most needed, preventing quality failures and ensuring regulatory compliance.
4. Quality/Technical Agreement (QTA): The QA Contract
The QTA is a formal document outlining the roles, responsibilities, and expectations between the pharmaceutical company and its suppliers.
Key Elements of a QTA
- Material release and testing responsibilities: It clarifies who performs final QC testing.
- Deviation and OOS/OOT handling: It ensures clear procedures for addressing quality events.
- CAPA timelines: Describe sets deadlines for corrective actions.
- Change notification and approval: Prevents unapproved changes that could impact quality.
- Documentation and record retention: Ensures compliance with regulatory inspection requirements.
QA teams are responsible for reviewing, approving, and maintaining QTAs, ensuring both parties remain accountable, compliant, and aligned with quality standards.
5. QA Tools for Supplier Oversight
Auditing is one of the most tangible ways QA verifies supplier reliability. It goes beyond document review. it offers direct insight into a supplier’s GMP culture, data integrity, and commitment to quality.
Types of Supplier Audits:
- Qualification Audit: Conducted before adding a new supplier to the approved list. Focuses on systems, controls, and compliance history.
- Routine (Surveillance) Audit: Ensures continuous compliance with GMP and internal quality expectations.
- For-Cause Audit: Triggered by specific events like deviations, customer complaints, or regulatory observations.
- Re-qualification Audit: Conducted periodically (usually every 2–3 years) based on supplier risk level.
During audits, QA evaluates:
- Facility layout and process flow
- Personnel training and hygiene
- Equipment calibration and maintenance
- Data integrity (ALCOA+ principles)
- Document control, batch records, and traceability
- CAPA effectiveness
Auditors document their findings using a graded classification such as Critical, Major, Minor, or Observation which determines follow-up timelines and risk rating.
Audit Follow-Up and CAPA
An audit doesn’t end when the report is issued. The most important part is ensuring the timely and verified closure of CAPAs. QA tracks:
- Root cause analysis quality
- Corrective and preventive measures
- Implementation evidence (photographs, SOP revisions, or training records)
- Effectiveness check results
Failure to close CAPAs on time can downgrade a supplier’s performance rating or even lead to temporary disqualification from the supply list.
Re-qualification:
Re-qualification ensures suppliers continue to meet current GMP standards and business requirements, reinforcing a culture of continuous improvement. It ensures long term consistency. QA may reassess documents and certifications, conduct follow-up audits & re-evaluate risk classification based on recent performance.
Consistent data tracking enables QA to rank suppliers as A (Excellent), B (Satisfactory) or C (Needs improvements) and focus attention on underperforming ones.
6. Documentation and Audit Readiness:
Documentation is the backbone of QA. Every activity from supplier evaluation to CAPA closure must be clearly recorded and readily retrievable.
Key Records
- Supplier qualification and requalification reports
- Approved supplier list (ASL)
- Audit plans, checklists, and reports
- CAPA follow-ups
- Change control documentation
- Supplier correspondence logs
During audits, regulators assess not only the presence of documents but also their accuracy, traceability, and timeliness. QA teams must therefore maintain document discipline, ensuring signatures, dates, and version control are always up to date.
7. Digital Transformation in QA:
The digital era has reshaped how QA manages suppliers. Traditional spreadsheets and manual tracking are being replaced by electronic Quality Management Systems (eQMS), AI-based analytics, and blockchain solutions.
Emerging Trends:
- Supplier portals: Secure sharing of documents and audit reports.
- Predictive analytics: Identify potential risks before they become issues.
- Blockchain: Ensure traceability, transparency, and authenticity of materials.
- Automation: Streamline audit scheduling, CAPA tracking, and trend analysis.
These innovations enable QA to move from reactive problem-solving to predictive quality management, aligning with Quality 4.0 principles.
8. Real-World Example:
A pharmaceutical company experienced repeated OOS results for a key excipient. Investigation revealed that the supplier had changed its raw material source without prior notification.

Corrective Actions
- Supplier suspended and requalified.
- QTA updated to mandate change notifications.
- Supplier risk level elevated, audit frequency increased.
Outcome
- Improved supplier communication
- Stronger adherence to quality agreements
- Zero recurrence of OOS results
This case highlights how proactive QA oversight prevents supply chain disruptions and ensures regulatory compliance.
9. The Future of QA in Supply Chain:
Effective supplier QA is not just about audits and monitoring; it’s about collaboration and mutual growth.
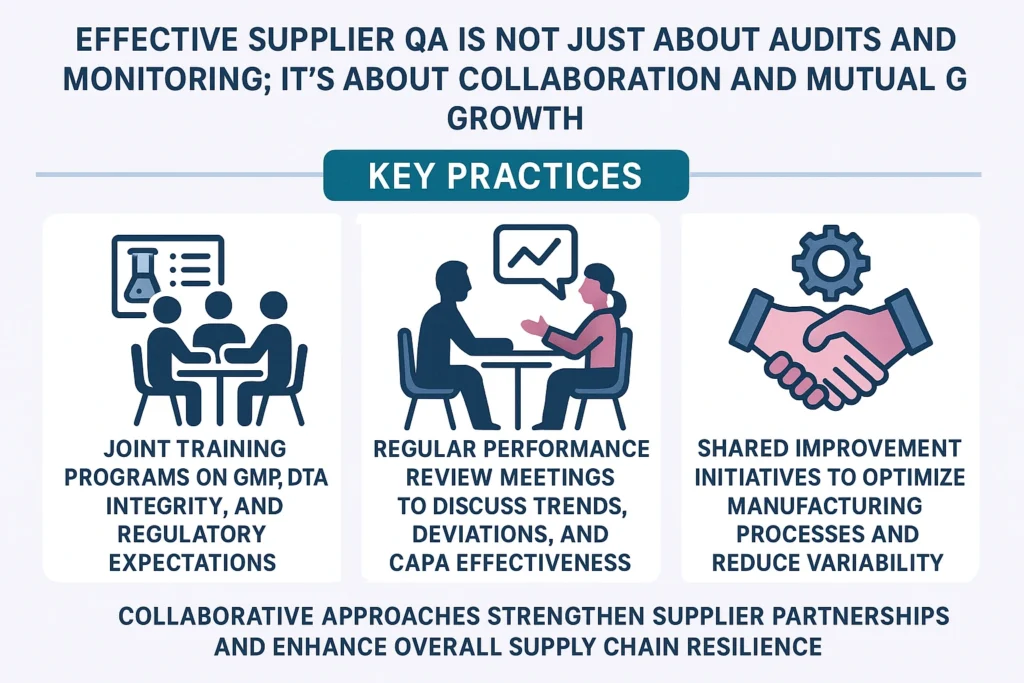
Key Practices
- Joint training programs on GMP, data integrity, and regulatory expectations.
- Regular performance review meetings to discuss trends, deviations, and CAPA effectiveness.
- Shared improvement initiatives to optimize manufacturing processes and reduce variability.
Collaborative approaches strengthen supplier partnerships and enhance overall supply chain resilience.
10. Continuous Improvement and Life cycle Management:
Supplier quality is not static, it evolves with market changes, new materials, and regulatory expectations. QA must therefore embed continuous improvement into its oversight model.
Continuous Improvement Activities
- Trend analysis of supplier deviations and audit results.
- Benchmarking against industry peers or best practices.
- Root cause evaluation of recurring issues and systemic fixes.
- Annual risk review to reclassify suppliers based on new data.
Companies adopting Plan–Do–Check–Act (PDCA) methodologies often experience measurable gains, including reduced rejections, fewer customer complaints, and improved supply continuity.
11. Flow Chart:
12. Training and Competency Development in QA Teams:
A robust supplier qualification program depends on competent QA professionals. The complexity of global supply chains requires expertise in both technical evaluation and strategic risk management.

Essential QA Competencies
- In-depth understanding of GMP and GDP guidelines.
- Skill in audit techniques and risk assessment tools (FMEA, HACCP, etc.).
- Awareness of international regulatory frameworks.
- Proficiency in digital quality systems and data analytics.
- Strong communication and negotiation skills for effective supplier collaboration.
QA departments should invest in ongoing training, internal workshops, and cross-functional learning to maintain global competitiveness.
13. Lessons Learned and Best Practices:
Successful pharmaceutical organizations share common habits in supplier quality assurance:
- Start qualification early in product development.
- Treat supplier audits as partnership dialogues, not inspections.
- Maintain a single source of truth for all supplier data.
- Reassess suppliers after any change in process, ownership, or location.
- Prioritize preventive action over corrective action.
By following these best practices, companies can significantly reduce supply disruptions and regulatory risks.
14. Conclusion:
Supplier qualification and risk management are essential components of pharmaceutical Quality Assurance, ensuring that only reliable, compliant, and GMP-aligned suppliers are part of the supply chain. By applying structured evaluations, risk-based tools, and continuous performance monitoring, QA helps prevent quality failures, supply disruptions, and potential patient-safety risks.
As global supply chains grow more complex, the role of QA is shifting from reactive oversight to proactive, data-driven risk control. Digital systems, automated workflows, and predictive analytics now support faster, more transparent decision-making throughout the supplier lifecycle.
A strong QA-led supplier management program ultimately protects product integrity, supports regulatory compliance, and strengthens long-term partnerships. In this evolving landscape, effective QA practices remain crucial to ensuring a safe, consistent, and resilient pharmaceutical supply chain.
References:
- European Commission (2022) EudraLex Volume 4: EU Guidelines for Good Manufacturing Practice for Medicinal Products for Human and Veterinary Use, Part I, Chapter 7 – Outsourced Activities. Available at: https://health.ec.europa.eu
- International Society for Pharmaceutical Engineering (ISPE) (2021) Good Practice Guide: Supplier Management. ISPE.
- International Council for Harmonisation (ICH) (2023) ICH Q9: Quality Risk Management. Available at: https://www.ich.org/page/quality-guidelines
- International Council for Harmonisation (ICH) (2008) ICH Q10: Pharmaceutical Quality System. Available at: https://www.ich.org/page/quality-guidelines
- World Health Organization (WHO) (2023) WHO Technical Report Series No. 986, Annex 2 – Good Manufacturing Practices for Pharmaceutical Products: Main Principles. Available at: https://www.who.int/publications
- U.S. Food and Drug Administration (FDA) (2006) Guidance for Industry: Quality Systems Approach to Pharmaceutical CGMP Regulations. Available at: https://www.fda.gov
- European Medicines Agency (EMA) (2018) Guideline on the Use of Risk Management Systems in Pharmaceutical Quality (EMA/INS/GMP/491213/2018). Available at: https://www.ema.europa.eu

