Introduction
High-Performance Liquid Chromatography (HPLC) is a valuable analytical technique that is used for the identification, separation, and quantification of various components in a mixture. The HPLC column is central to this technique, functioning to separate the analytes.

Stationary phase VS Mobile phase
The stationary phase of the column is a solid component that interacts with the components in the sample, while the mobile phase is a liquid that facilitates the passage of the sample through the column. The different nature of the interactions between the analytes and the stationary phase is responsible for the separation. Knowing the different properties of HPLC columns is important for selecting an appropriate column for each method, but it is also important for achieving the optimal separation.
This article will discuss HPLC columns, packing materials, physical and chemical properties, and basic silica chemistry.
HPLC Column Construction and Packing Materials
A column intended for high-performance liquid chromatography (HPLC) contains several different components, all of which require a high degree of pressure (thousands of psi) and must promote a uniform flow path for the liquid mobile phase. The primary components of an HPLC column include the column tube, end fittings, frits, and packing material.
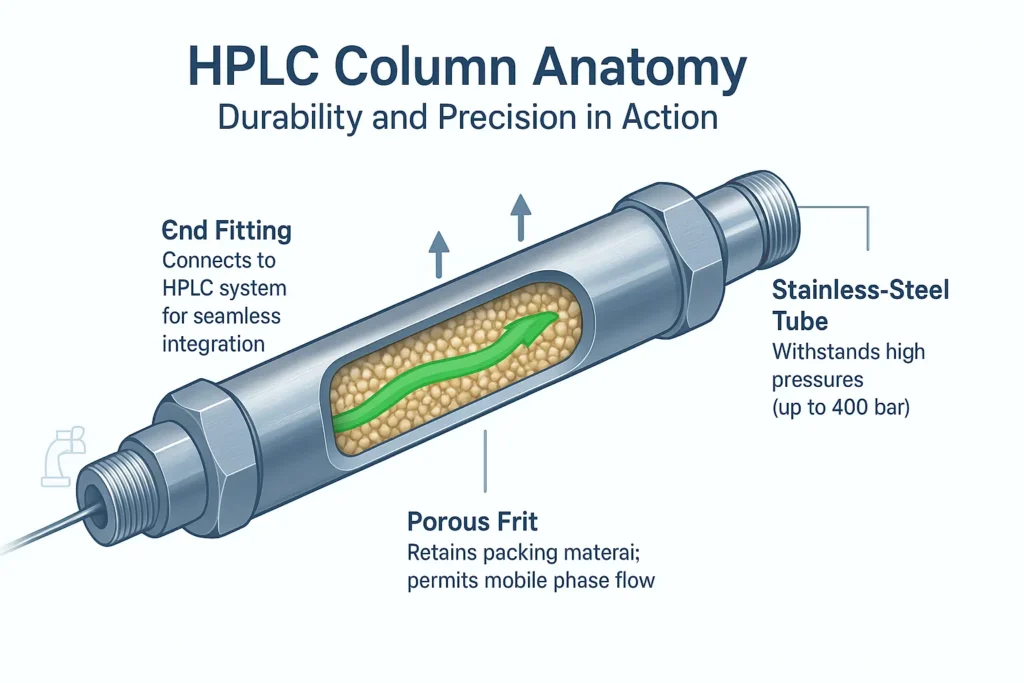
HPLC column tubes are typically composed of stainless-steel material to withstand the high pressures that can occur during HPLC operation. The end fitting connects the column to the HPLC system, while frits are porous discs that hold the packing material in the column, allowing the mobile phase to pass through.
💡 Technical Note
The packing, or stationary phase, is certainly the most important component of the HPLC column as it determines the separation mechanism. HPLC packing materials are generally divided into two categories, which are classified as normal-phase and reversed-phase.
Normal phase vs. Reversed-phase chromatography
Normal Phase Chromatography:
A polar stationary phase and a non-polar mobile phase characterize normal phase chromatography. This style of chromatography is appropriate for separating polar analytes. Silica gel is the most widely used packing material for normal phase columns; such silica gel can be either amorphous or spherical in shape and usually has particle sizes of between 3 and 10 µm.
The surface of the silica gel contains silanol groups (-Si-OH) that are polar and interact with polar analytes. Another form of packed silica gel utilized in normal phase chromatography, bonded phase silica gel, chemically bonds functional groups such as cyano (-CN) or amino (- NH2) to the silica surface to provide unique selectivity.
Reversed Phase Chromatography:
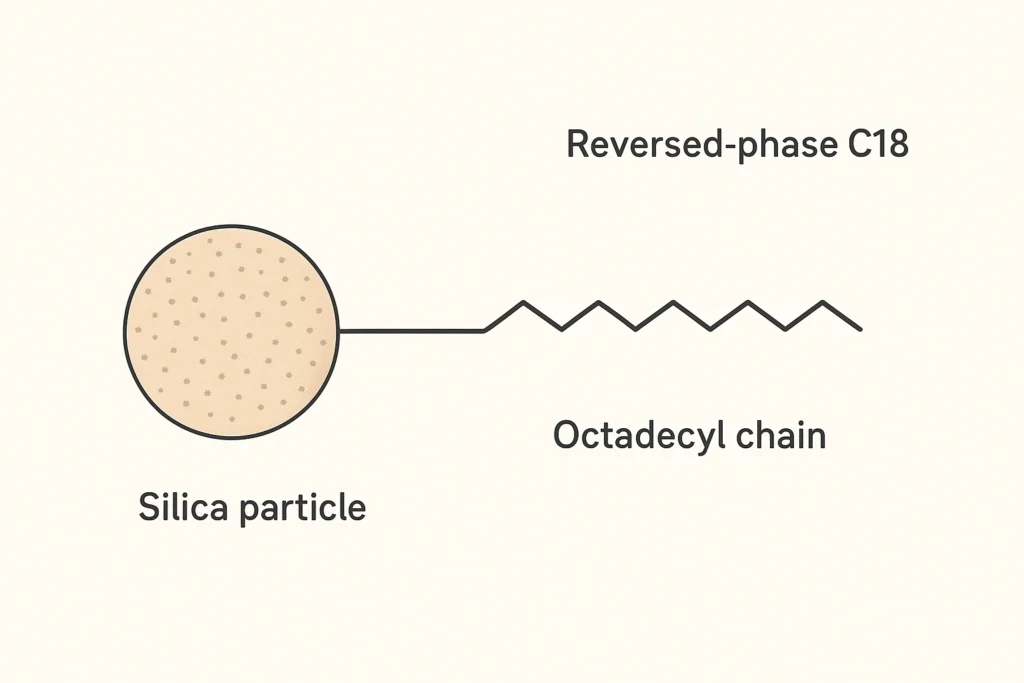
Reversed-phase chromatography is the most commonly used mode of HPLC. In the reversed mode, the stationary phase is non-polar and the mobile phase is polar (normally a mixture of water and the organic solvent acetonitrile or methanol). Reversed-phase chromatography is suitable for separating nonpolar and moderately polar compounds. The most common reversed-phase packing is silica gel that has been chemically modified to add non-polar functional groups. The most common bonded phase is the octadecyl (C18) group for reversed-phase chromatography. Other common bonded phases for reversed-phase chromatography include octyl (C8), butyl (C4), and phenyl groups, among others. These types of bonded phases create a non-polar surface that interacts with non-polar analytes through hydrophobic regions.
Key Physical and Chemical Properties:
The performance of an HPLC column is influenced by a combination of some physical and some chemical characteristics of the packing material, with the particle size, pore size, surface area, and carbon load being the most important. Knowledge of these factors is important to select the proper column for a specific separation.
1-Particle Size
The particle size of the packing material is the most important factor affecting the efficiency and the back pressure of the HPLC column.
💡 Technical Note
Smaller stationary-phase particles provide a higher surface area-to-volume ratio, leading to more efficient separations. However, because they pack more densely, they also generate greater back pressure. Particle size is usually reported as an average diameter, and a narrow size distribution helps maintain a uniform and stable packed bed.
D10/90 ratio:
The D10/90 ratio, which is the particle size at the 10th and 90th percentiles of the distribution, is sometimes reported as an indication of the range of the particle size distribution. A D10/90 ratio of 1.2 or less is considered very good.
When measuring a HPLC packing material’s (stationary phase) particle size distribution, the distribution will generally be presented in the form of a cumulative distribution curve, which will show the percentage of total particles that would fall below a given size.
- D10: the particle diameter below which 10% of the particles will fall. → So, 10% of the particles are smaller than D10.
- D90: the particle diameter below which 90% of the particles will fall. → Therefore, 10% is larger than D90, or 90% is smaller than D90.
This means that a lower ratio is preferred since it means a narrower distribution, meaning the particles are nearly the same size, meaning very uniform. In comparison, a higher ratio would mean a broader distribution, meaning there is a bigger spread of particle sizes, thus less uniform.
To summarize the above, a low D10/D90 ratio indicates a tighter packing, meaning fewer voids or channels inside the bed, also it indicates a consistent flow path of mobile phase, thus ensuring less band broadening, therefore getting sharper peaks. Moreover, a uniform particle size would achieve reproducibility between different column Lot numbers.
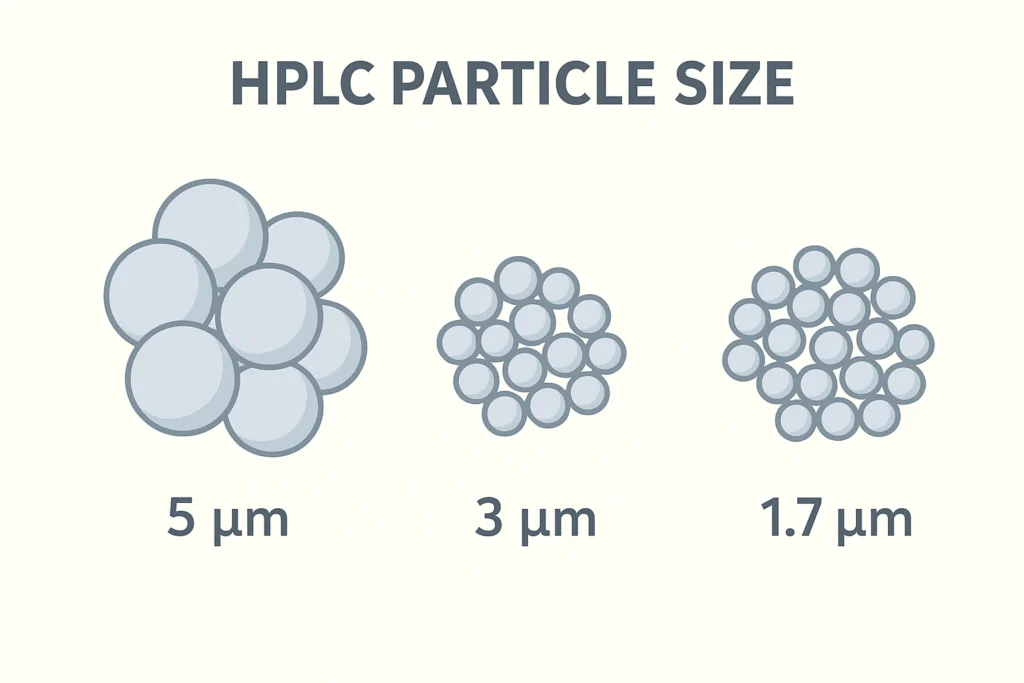
- Sub-2 µm particles: Sub-2 µm particles are used in Ultra-High-Performance Liquid Chromatography (UHPLC) for either extremely high-resolution separations or very fast separations. These particles require specialized UHPLC systems because they must operate at very high pressures.
- 2-5 µm particles: These are considered the “standard” particle sizes used for less complex separations in standard HPLC applications. The 2-5 µm particles balance efficiency and back pressure well.
- >5 µm particles: Larger particles are used for preparative chromatography, which focuses on purifying large quantities of material, and less complex analytical separations
D10 → 10% of particles are smaller than this point.
D50 → The median particle size.
D90 → 90% of particles are smaller than this point.
👉If D90/D10 ≈ 1.2 → particles are nearly the same size → uniform packing, smooth flow, sharp peaks.
⚠️ If D90/D10 is large → wide spread in sizes → uneven packing, voids, broader peaks.
In summary, A low D10/D90 ratio means tighter, more consistent packing and better chromatographic performance.
2-Pore Size
The pores in the silica particles provide the surface area for the stationary phase and engage in the separation process. The pore size of the packing material is important to the accessibility of the analyte molecules to the stationary phase. A general rule of thumb is that the pore diameter should be a minimum of three times smaller than the hydrodynamic diameter of the analyte molecule to facilitate unrestricted access to the pores of the stationary phase. The pore size is typically measured in angstroms (Å).
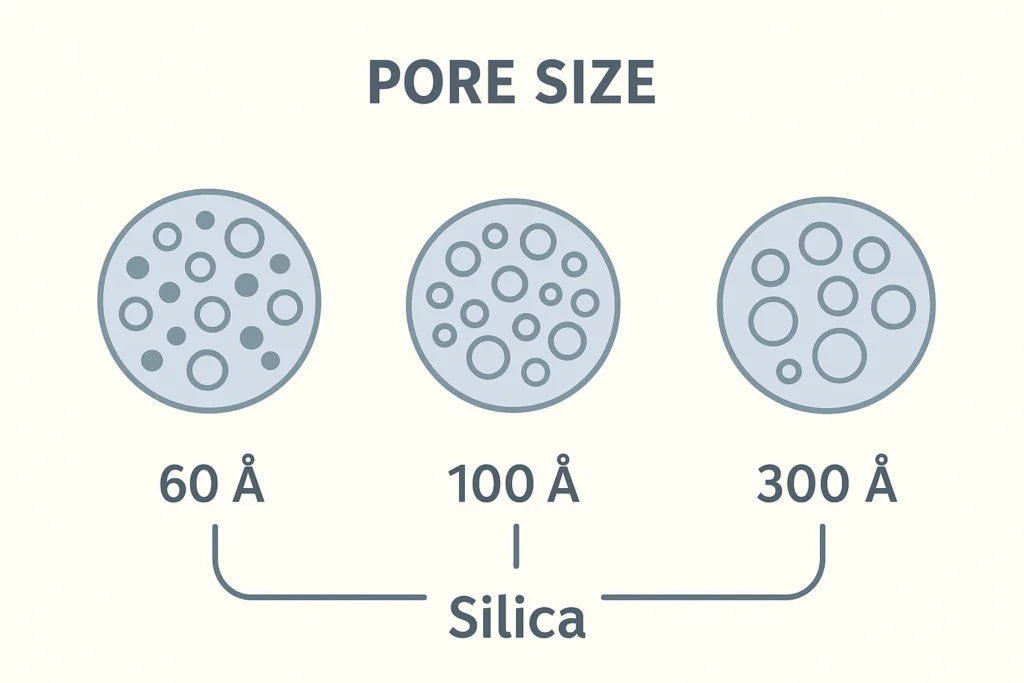
- <100 Å pores: These are considered narrow pores and are suitable for the separation of small molecules (typically < 3,000 Da).
- 100-130 Å pores: These are intermediate pore sizes and are suitable for the separation of molecules in the range of 3,000-10,000 Da.
- >130 Å pores: These are considered large pores, and are used for the separation of larger molecules, such as peptides and proteins (>10,000 Da).
3-Surface Area
The surface area of the packing material describes the total area available to interact with the analyte molecules. Typically, a larger surface area will lead to greater retention and also greater sample loading capacity. Surface area is generally expressed in m²/g (square meters per gram). HPLC packing surfaces typically have a surface area that ranges from 100 to 400 m²/g. The surface area is inversely proportional to the size of the pore; larger pores will have smaller surface areas.
4-Carbon Load
For reversed-phase columns, the carbon load expresses the amount of carbon bonded phase that has been bonded onto the silica surface, or it can be stated as a % by weight of carbon of the material. The carbon load is a key parameter that determines the hydrophobicity of the stationary phase.
Generally, the more carbon that is bonded to the silica bed, the more hydrophobic the stationary phase, and the retention of non-polar analytes typically increases with a greater carbon load. Carbon load is determined by gravimetric analysis, where the packing material is weighed before and after the bonded phase is burned off in a muffle furnace
- Low Carbon Load (e.g., 7%): This leads to a stationary phase that is less hydrophobic and faster elution of non-polar analytes.
- High Carbon Load (e.g., 15-30%): This leads to a stationary phase that is more hydrophobic and stronger retention with higher sample capacity
5-End Capping
After primary bonding of the stationary phase to the silica surface, typically, there are residual silanol groups that haven’t reacted. The residual silanols can be acidic and will interact with basic analytes, resulting in peak tailing and other undesirable effects in chromatography. End capping is a secondary chemical reaction that can occur, which is meant to cap the residual silanol groups with a small non-polar group, typically a trimethylsilyl (TMS) group. End capping will result in decreasing the activity of the residual silanols and improve the overall performance of the column, especially in the presence of basic compounds. Double or even triple End capping can also be performed to further decrease the activity of residual silanols.
6-Silica Chemistry
The chemistry of the silica support has a major effect on the performance of the HPLC column. The kind of silica can affect the purity, stability, and selectivity of the stationary phase.
Silica Types:
- Type A Silica: This is the older, traditional silica that was used in the early days of HPLC. It is associated with higher levels of metal content and a more acidic surface chemistry, and this can lead to poor peak shape for base compounds.
- Type B Silica: This is a high-purity silica that is specially processed to remove the metal content and form a more homogenous surface. This is the most common form of silica in standard HPLC columns and performs well for many applications.
- Type C Silica: This is a new type of silica and has a hydride (Si-H) surface, as opposed to the more common silanol (Si-OH) surface. The different surface chemistry provides different selectivity than standard silica columns and has some advantages for certain applications.
7-Bonded Phase Chemistry
The type of the bonded phase defines the dominant separation mechanism from the HPLC column. Generally, the most widespread bonded phases are based on organosilanes spliced into the silica surface of a liquid chromatographic column. The bonded phase selected depends on the properties of the analytes to be separated.
- C18 (Octadecyl): The most common reversed-phase material and presents which provides the strongest hydrophobic retention.
- C8 (Octyl): Less hydrophobic than C18, and provides shorter retention times and alternative selectivity.
- Phenyl: Unique selectivity enhancement for aromatic and unsaturated compounds, about pi-pi interactions.
- Cyano (CN): Can be used for both normal-phase and reversed-phase, but elutes with a different selectivity than alkyl or phenyl phases.
- Polar-Embedded Phases: These phases will have a polar functional group (e.g., amide, carbamate) embedded into the alkyl chain.
- The polar functional group inhibits interaction with the residual silanols and will give alternative selectivity for polar compounds, about column chemistry or the bonded phase.
Conclusion
The HPLC column is a critical component of the HPLC system. An understanding of the various characteristics of HPLC columns, including packing material, particle size, pore size, surface area, carbon load, and silica chemistry, is important to select the proper column and develop robust and reproducible HPLC methods. If chromatographers are effective at accounting for these column characteristics, they should be able to achieve effective separation performance through a variety of analytical applications.
References
- http://The LCGC Blog: Silica for HPLC Stationary Phases – A Five Minute Guide
- http://HPLC Columns Packing – Hawach
- http://HPLC Columns: Carbon Load and Surface Area Explained – Axion Labs
- http://The LCGC Blog: Things You Should Know About Your HPLC Column, Part I – Pore Sizes and Particle Diameters
- http://What Is Endcapping in HPLC Columns – Chrom Tech, Inc.
- http://What Is on Your HPLC Particle? A Look at Stationary Phase Chemistry Synthesis



I have been surfing online more than three hours today, yet I never found any interesting article like yours. It is pretty worth enough for me. In my view, if all site owners and bloggers made good content as you did, the net will be much more useful than ever before.