In the highly precise and regulated world of pharmaceutical manufacturing, the transformation of fine powders into robust, uniform granules is a critical step. This process, known as granulation, is the alchemy that turns a therapeutic formula into a manufacturable product.
We have already included a very interesting, detailed discussion about granulation basics in this series of articles. Please follow the button below for more information:
For decades, the most common method in this field has been wet granulation, a technique that depends on liquid binders. However, a compelling alternative has been steadily gaining prominence, offering a paradigm shift away from solvents and towards thermal energy: Melt Granulation.
Melt granulation, also known as thermoplastic granulation or hot melt granulation (HMG), is a one-pot process where powdered API (Active Pharmaceutical Ingredient) and excipients are agglomerated using a molten binder. Instead of adding a liquid that must later be dried, the binder itself is melted within the mixture, coating the particles and, upon cooling and solidification, forming strong solid bridges that create durable granules. This method represents a fundamental rethinking of particle engineering with profound implications for efficiency, stability, and product performance.
The Fundamental Principle: Solid Bridges from a Liquid State
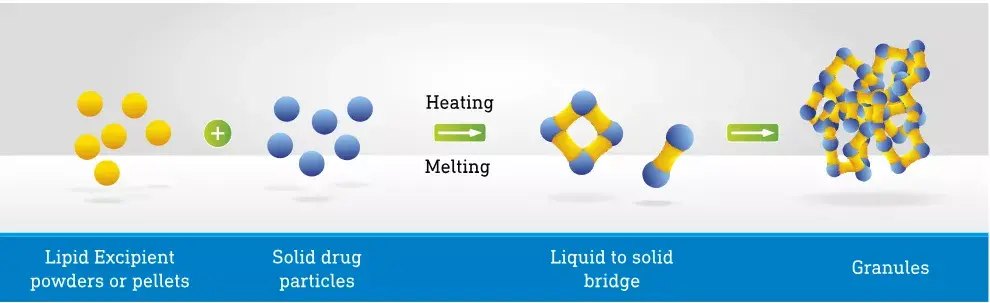
At its core, melt granulation is deceptively simple. It leverages a class of excipients known as meltable binders. These are typically waxy or polymeric solids at room temperature but possess a relatively low melting point, often between 50°C and 80°C. Common examples include polyethylene glycols (PEGs) , poloxamers, glycerides (e.g., Gelucire series), and certain surfactants.
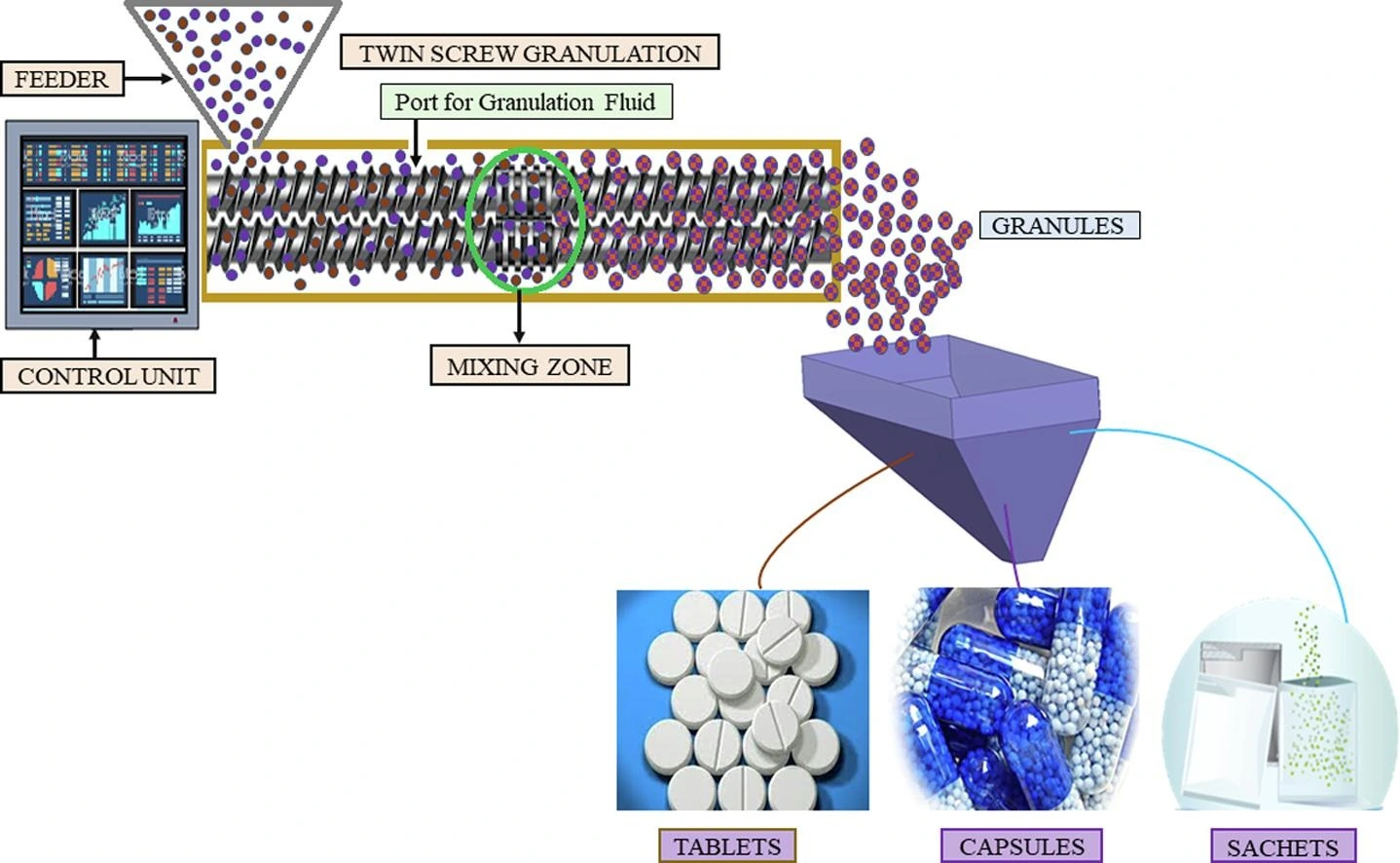
The process unfolds in a high-shear granulator or, increasingly, in a twin-screw extruder. The powder blend is agitated and heated, either by a jacketed vessel or through frictional and shear forces generated by the impellers and choppers. Once the temperature exceeds the melting point of the binder, it transitions into a viscous liquid. This molten binder then acts as the granulating liquid, distributing itself throughout the powder bed.
The resulting shear forces, which are rather intense, can definitely ensure coating of the primary particles, resulting in nucleation, followed by coalescence and growth into granules. The final, crucial step is cooling. As the mass cools, the binder solidifies, forming the solid bridges that interlock the particles together. The resulting granules exhibit excellent flow properties, uniform content, and high mechanical strength.
The Distinct Advantages of HMG
The advantages of HMG are significant and multifaceted:
- Solvent-Free Nature: This is the most profound advantage. The release of water or organic solvents eliminates the entire, costly, and time-consuming drying step (typically done in a fluidized bed dryer). This significantly reduces time and energy consumption. More importantly, it eliminates any risk of solvent residue in the final product, a critical quality attribute. It also renders the process inherently safe for moisture-sensitive or hydrolytically unstable APIs, which can degrade in the presence of water.
- Single-Pot Processing: Melt granulation can often be completed in a single high-shear granulator. The entire sequence—mixing, heating/granulating, and cooling—can occur without transferring the product. This minimizes material handling, reduces the risk of cross-contamination, and simplifies cleaning validation. It aligns perfectly with the pharmaceutical industry’s push towards continuous manufacturing, as twin-screw melt granulation is an excellent continuous process.
- Enhanced Stability and Controlled Release: The hydrophobic nature of many meltable binders (like glyceryl behenate, or Compritol) can create a protective matrix around the API. This can result in a protective barrier from atmospheric oxygen and moisture, enhancing chemical stability. Also, by choosing the appropriate waxy binders and altering process parameters, necessary modifications can be easily applied to granules for specific drug release profiles, such as sustained or delayed release, without the need for additional polymer coatings.
- Improved Content Uniformity: The homogeneous distribution of the molten binder, which often contains a dissolved or dispersed API, can lead to superior content uniformity compared to dry mixing, especially for low-dose, high-potency drugs where homogeneity is a major challenge.
Critical Process Parameters and Material Attributes
The process requires a delicate balance of thermodynamics and rheology, where success is dictated by a deep understanding of both process parameters and material attributes.
Key Critical Process Parameters (CPPs):
- Product Temperature: This should be high enough to fully melt the binder but controlled to prevent overwetting, which leads to excessive granule growth and the formation of a pasty mass. Low temperature results in incomplete melting and poor granulation.
- Impeller and Chopper Speed: Shear forces are the engine of distribution and consolidation. Higher speeds increase the collision frequency and energy, leading to denser, smaller granules. Lower speeds may produce larger, more porous agglomerates.
- Heating/Cooling Rate: The rate at which the mass is heated and, crucially, cooled can influence the crystalline structure of the solidified binder, which in turn affects granule strength and drug release properties.
- Process Time: The duration of the molten state (kneading time) impacts granule consolidation and growth.
Key Critical Material Attributes (CMAs):
- Binder Properties: The melting point, viscosity in the molten state, and surface tension of the binder are paramount. A low-viscosity melt will spread more easily, while a high-viscosity melt may lead to uneven distribution. The binder’s hydrophobicity dictates drug release.
- API Properties: The thermal stability of the API is non-negotiable. It must withstand the processing temperatures without degradation. The API’s solubility in the molten binder can affect the granulation mechanism and drug release.
- Particle Size and Hydrophobicity of Fillers: The size and surface characteristics influence how readily they are wetted by the molten binder.
The interplay between these factors is complex. For instance, an increase in impeller speed can compensate for a higher viscosity binder by providing more distributive energy. This necessitates a Quality by Design (QbD) approach, where the design space—the combination of material attributes and process parameters that assures quality—is systematically mapped out.
Emerging Applications and Advanced Techniques
Melt granulation is not a static technology; it is a platform for innovation. Its application is expanding beyond simple granulation for tableting into more advanced territories:
- Twin-Screw Melt Granulation (TSMG): The adaptation of melt granulation to twin-screw extruders is a game-changer. It transforms the process from batch to continuous, offering superior scalability, better heat and mass transfer, and precise control over residence time. TSMG is a cornerstone of the modern, continuous oral solid dosage manufacturing plant.
- Formulation of Poorly Water-Soluble Drugs: This is a particularly exciting frontier. Many new chemical entities suffer from poor aqueous solubility, limiting their absorption. The Melt Granulation can be used to create Solid Dispersions, where the API is dissolved in the molten hydrophilic polymer (e.g., a poloxamer or PEG) and, upon cooling, is trapped in an amorphous state within the polymer matrix. This amorphous solid dispersion can dramatically enhance the dissolution rate and oral bioavailability of the drug. In this context, the granulator or extruder becomes a reactor for creating the drug’s final, performance-optimized physical form.
- Taste Masking: For pediatric or geriatric formulations, unpleasant taste is a major compliance issue. By embedding a bitter API within a waxy, inert matrix that is insoluble in saliva but dissolves in the gastric fluid, melt granulation can effectively mask taste without the need for complex coating processes.
Navigating the Challenges
Despite its considerable advantages, melt granulation is not a universal panacea. It presents unique challenges that require careful consideration:
- Thermal Stress: The primary limitation. The API and all excipients must be stable at the processing temperature, which can be a barrier for thermolabile compounds.
- Binder Selection: The palette of pharmaceutically approved, low-melting-point binders, while growing, is still more limited than the range of solvents and binders available for wet granulation.
- Energy Input: The process requires significant energy for heating, and controlled cooling is essential, which can be a technical challenge in some equipment.
- Potential for Polymorphic Transitions: The heating and cooling cycles can induce changes in the crystalline form (polymorphism) of either the API or the binder, which must be meticulously characterized as it can alter stability and performance.
Conclusion
Melt granulation has firmly established itself as a powerful and versatile tool in the pharmaceutical engineer’s arsenal. It is a testament to the industry’s evolution towards more efficient, sustainable, and sophisticated manufacturing paradigms. By replacing solvents with thermal energy, it addresses critical issues of time, cost, and stability. Its synergy with continuous manufacturing and its application in solving modern formulation challenges like bioavailability enhancement position it at the forefront of pharmaceutical innovation.
As our understanding of material science deepens and equipment design becomes more advanced, the boundaries of melt granulation will continue to expand.