Definition of the term “Buffer Solution”
Buffer Selection is the process of selecting a buffer solution, which can resist a change in pH when an acid or base in minor quantities is added. The buffer solution is made of an acid and its conjugate base or vice versa. The main aim of a buffer solution is to neutralize the acid or base being added to avoid the sudden change in pH.
The mechanism by which a buffer acts is to react with either the hydroxide ions or the hydrogen ions, blocking them from affecting the pH at their full potential. However, it should be mentioned that in general buffer solutions have a limit regarding their ability to neutralize before the buffer solution reaches its full capacity, after reaching such maximum capacity, the pH of the solution will begin to change as if no buffer is found at all.
pH, pKa, and UV cut-off
Buffer capacity
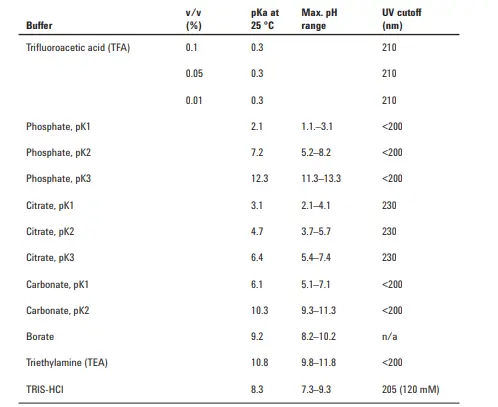
Choosing the right buffer depends mainly on the buffer characteristics of pKa, pH range, UV cutoff, and pKa of the drug or API to be separated.
- As a rule, a buffer should be used for a pH that is within +/-1 units of its pKa value, within such a range.
- The buffer can resist any deliberate change in pH.
- Full buffer capacity is observed when its pH is equal to its pKa.
- The high buffer capacity is achieved when a high concentration of acid/conjugate base or base/conjugate acid is used.
UV Cut-off
UV cut-off value should also be considered to avoid any interference of the detection wavelength with the buffer absorbance. For example, a significant absorbance is observed for TFA (Trifluoroacetic acid) at <220 nm and for both formic acid and acetic acid at <240 nm.
It is very recommended for an ionizable analyte buffer selection pH to be at least 2 units away from the pKa of the analyte, otherwise, split peaks might be observed for the analyte due to the current presence of both ionized & non-ionized states for the analyte.
Buffer selection and its relation to the HPLC column selection
Generally, for Buffer selection, a buffer concentration of 10-50 mM is suitable for small molecules. The buffer’s concentration should be the lowest concentration needed to give reproducible results, since a very high concentration of a buffer could lead to its precipitation, causing HPLC column back pressure and deterioration over time. For a reversed-phase HPLC column, the most ideal pH range is from pH 2 to 8.
A pH less than 2 would cause bonded phase loss, while a pH higher than 8 would cause silica deterioration. In general, in HPLC columns, silica of high purity is much more able to tolerate higher pHs than that of low purity.
Silanol groups on the silica surface of the column have a potential ionization which would affect the retention of acidic and basic analytes where usually Silanol groups would be deprotonated meaning negatively charged, accordingly, it would cause ion-exchange interaction greater retention- with positively charged ie basic ions, this secondary interaction is unfavored since it would result in peak tailing or peak broadening.
Buffer Molarity
The aim is to use the lowest concentration of a buffer, a concentration that can give a reproducible result. In general, the buffer concentration should not be less than 5mM, otherwise, the solution prepared will not be as effective as a buffer (depending on the buffering capability & the sample concentration). Moreover, raising the buffer concentration higher than 100 mM is risky where buffer precipitation (salting out) could occur leading to increasing the HPLC column back pressure, blockage, and overtime deterioration of the HPLC column, also such a high concentration for the buffer solution increases the rate of wearing out for the HPLC pump movable part that’s why a back-seal wash is very recommended to be installed.
Buffer Solubility
A buffer should be completely water soluble, and adding to this, it should not precipitate during analysis when being added or mixed with a chosen organic solvent. As a general rule, a percentage of more than 50% organic shouldn’t be used or mixed with a buffer; this would depend on the type of buffer as well as the buffer’s concentration. So, to summarize the buffer selection, the buffer solution should be clear, homogenous, and particulate-free. If the buffer solution is stored, it should be considered that buffers have a limited lifetime, so it is favorable for any buffer solution to be prepared daily.
Types of salts
For HPLC with UV detection, the most common buffers used are phosphate buffers & acetate buffers. That\’s why both buffers are extremely useful because they can be used at wavelengths <220 nm. From the above table attached, it is observed that phosphate has three pKa values that would correspondingly give three buffering ranges:
1- pH range: (1.1 – 3.1).
2- pH range: (6.2 – 8.2).
3- pH range: (11.3 – 13.3)
So, generally, we use phosphate buffer at range (2-3) (6.2 – 8.2) otherwise the rest is practically unaccepted to avoid deterioration of the HPLC column.
According to the previously mentioned pKa range for phosphate, it is observed that there is a gap for phosphate in buffering between the range of pH 3.1 to pH 6.2; filling this gap is possible when using acetate, where it has a buffering range of (3.8 – 5.8). This pH is also favorable for most HPLC columns to operate with.
Sometimes in method development, covering the whole pH range of an HPLC column (pH 2-8) is needed to have full control of the pH over such a useful range for the HPLC column, in such cases a blend of phosphate & acetate buffer will allow the continuous variation of the mobile phase along the pH range of the column (pH 2-8). The moment you find a desired pH, one of the two salts of acetate or phosphate can be excluded by then, meaning, for example if the final mobile phase is 4.5 then in such case, acetate is only needed, ie, phosphate salt is not needed to be used at all in this case.
The analysts sometimes favor Citrate since it has three overlapping pKas covering the range of (2.1 – 6.4). Yet, citrate is not favored many times since it has a high cut-off, which would make it very challenging to use at wavelengths <220 nm; also, many problems appear in HPLC check valves after citrate usage.


