Abstract

Quality in the life sciences is often mistaken for compliance: having the right SOPs, passing inspections, or closing CAPAs on time. Yet, history shows that firms can be fully “compliant” on paper and still fail patients. This has prompted regulators worldwide — including the FDA, EMA, MHRA, and WHO — to emphasize the importance of quality culture. A culture of quality goes beyond documentation and procedures; it reflects the values, behaviors, and mindsets of an organization.
This article explores the distinction between compliance and culture, presents regulatory perspectives, and explores case studies of cultural success and failure, ultimately proposing a practical roadmap for embedding quality into the DNA of organizations involved in pharmaceutical manufacturing and clinical research.
Introduction: Why Compliance Alone Cannot Protect Patients
The pharmaceutical and clinical research industries are among the most highly regulated sectors in the world. Despite this, high-profile compliance failures continue to dominate headlines.
In 2023, the U.S. Food and Drug Administration (FDA) issued over 480 Form 483 observations. Many cited issues were not due to absent SOPs but to weaknesses in data integrity, documentation practices, and cultural attitudes toward quality. Similarly, the European Medicines Agency (EMA) and the UK Medicines and Healthcare products Regulatory Agency (MHRA) have repeatedly identified “tick-box compliance” as a root cause of systemic risk.
In clinical research, the stakes are equally high. Trials that fail to embed quality culture risk poor data reliability, protocol deviations, and ultimately the safety of participants. In manufacturing, poor culture can trigger batch failures, product shortages, recalls, and regulatory sanctions.
The lesson is clear: compliance is necessary, but not sufficient. What regulators, patients, and industry now demand is culture.
Compliance vs. Quality Culture: Understanding the Divide
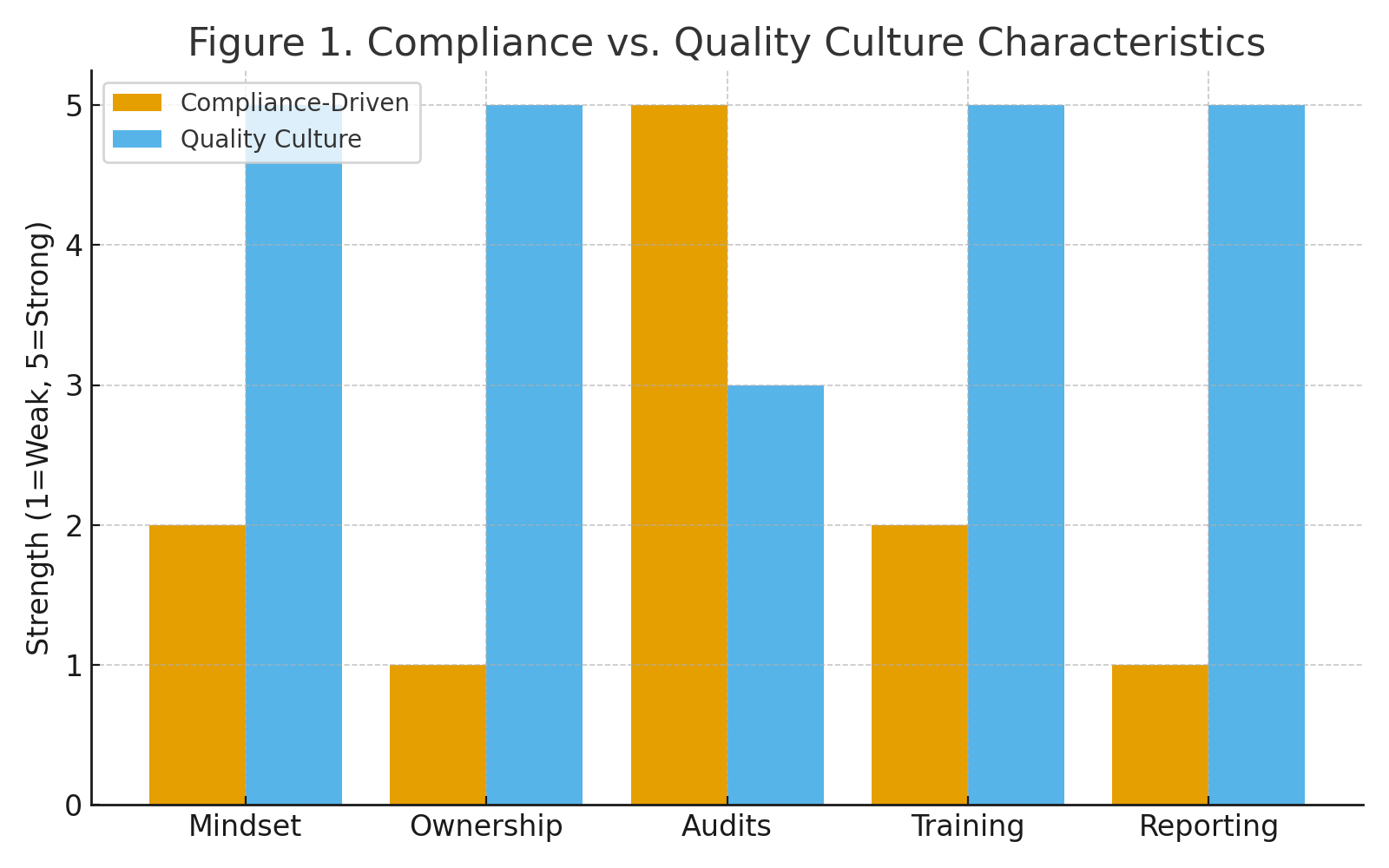
The terms “compliance” and “quality” are often used interchangeably, but they are not the same. Compliance refers to adherence to external requirements: laws, regulations, and standards. Quality culture, on the other hand, is internal. It reflects how people think, act, and make decisions when no inspector is watching.
Table 1. Compliance-Driven vs. Culture-Driven Approaches
| Compliance-Driven | Quality Culture |
|---|---|
| We follow the SOP because the auditor will ask.” | We improve the SOP because patients depend on us.” |
| QA holds responsibility for quality. | Every function shares responsibility for quality. |
| Focus on passing inspections. | Focus on continuous improvement and patient safety. |
| Training is a checkbox. | Training is experiential, practical, and ongoing. |
| Issues are hidden until they are forced into the open. | Issues are escalated early without fear of reprisal. |
See also Pharmacovigilance, Drug Safety Monitoring
The Global Regulatory Push Toward Quality Culture
Over the past decade, regulators have signaled a consistent message: culture matters as much as compliance.
- FDA (U.S.): Through its Quality Metrics Initiative, the FDA encourages companies to track leading indicators of cultural strength, such as deviation reporting rates, right-first-time performance, and employee engagement.
- EMA (Europe): ICH Q10 (Pharmaceutical Quality System) underscores management responsibility for building a quality culture, embedding it alongside technical system requirements.
- MHRA (U.K.): Introduced GXP Culture Maturity Models, assessing openness, transparency, and staff willingness to escalate concerns.
- WHO: Emphasizes that sustainable quality cannot be achieved through procedures alone; cultural attitudes are essential to uphold global GMP standards.
These initiatives illustrate a shift: regulators are no longer satisfied with SOPs and CAPA logs. They want to see evidence of behaviors, decision-making, and leadership commitment to quality.
Why Quality Culture Breaks Down
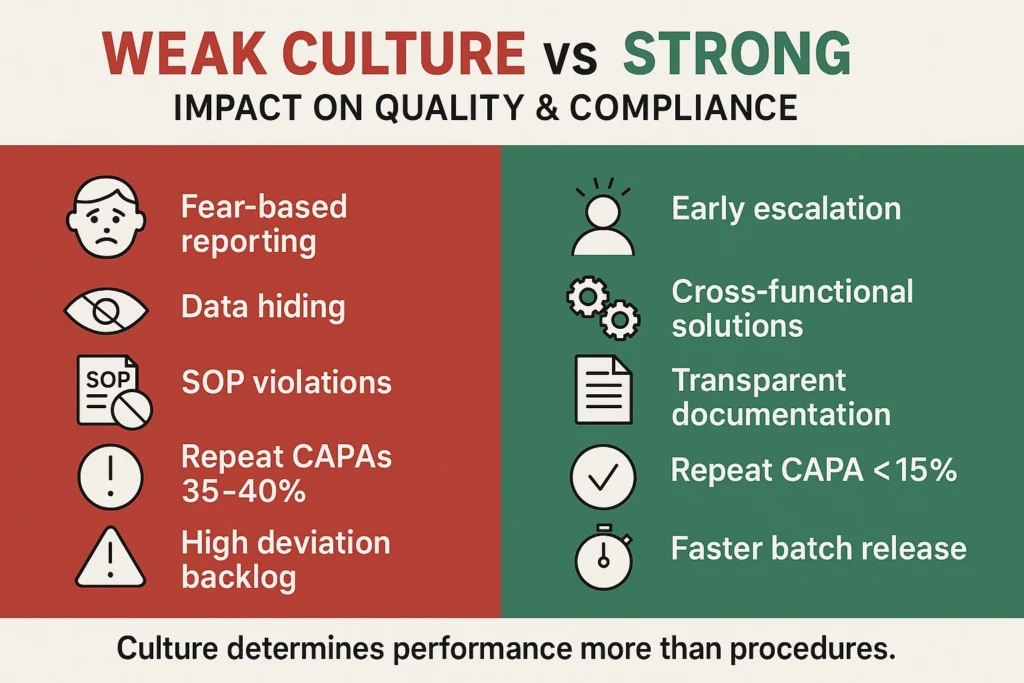
Despite good intentions, many organizations fall short. Common pitfalls include:
- Leadership Disconnect
- Executives focus on cost and timelines, leaving QA as the “guardian of quality.”
- This creates misalignment between business objectives and patient safety.
- Fear of Reporting
- In cultures where employees fear blame, errors are hidden rather than escalated.
- This creates blind spots that auditors eventually uncover.
- Audit Dependence
- Organizations rely on external inspectors to find issues rather than building self-identification mechanisms.
- Training Fatigue
- Annual GMP/GCP slide decks create disengagement. Staff “pass” training but fail to internalize quality principles.
Measuring the Cost of Weak Culture
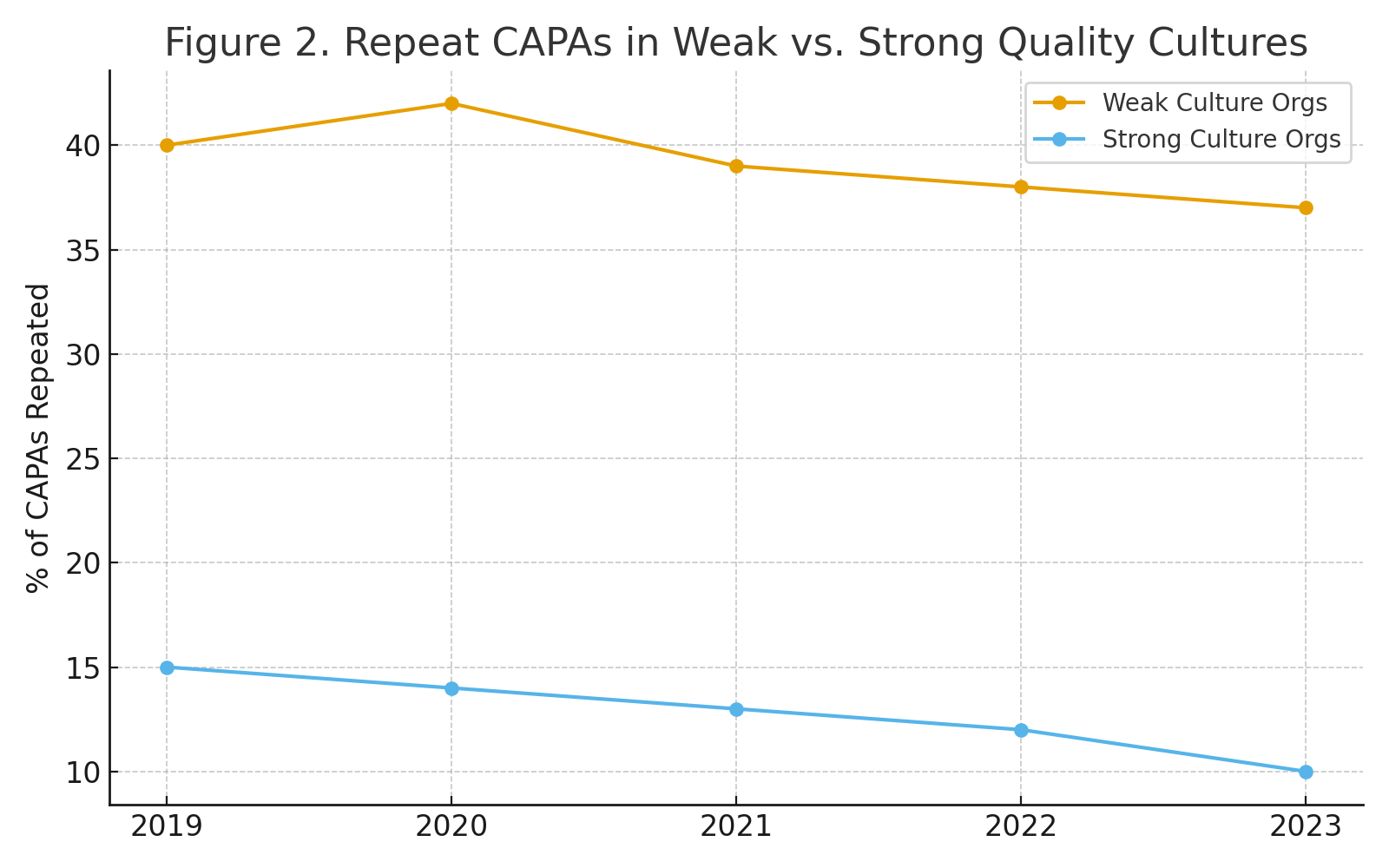
Organizations with weak quality culture report repeat CAPAs in 35–40% of cases, reflecting superficial fixes. In contrast, organizations with strong culture reduce repeat CAPAs to 10–15%, demonstrating systemic problem-solving.
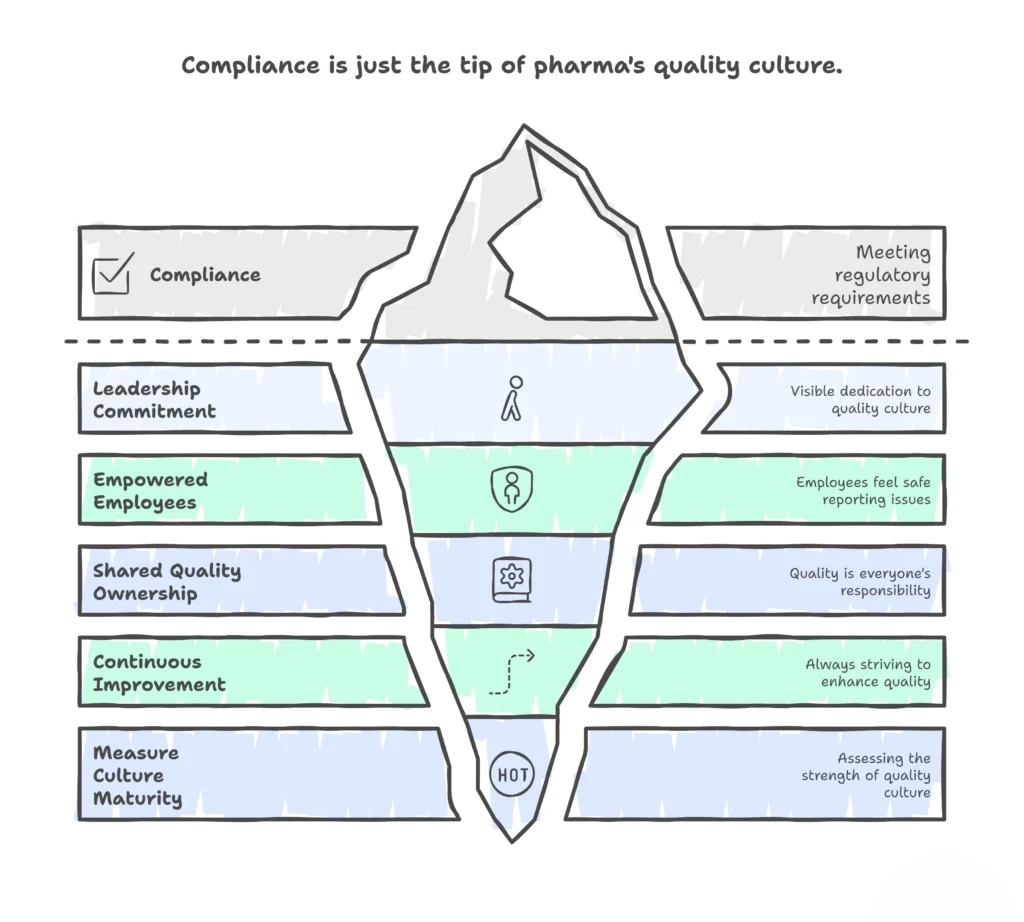
Building a True Quality Culture: A Practical Roadmap
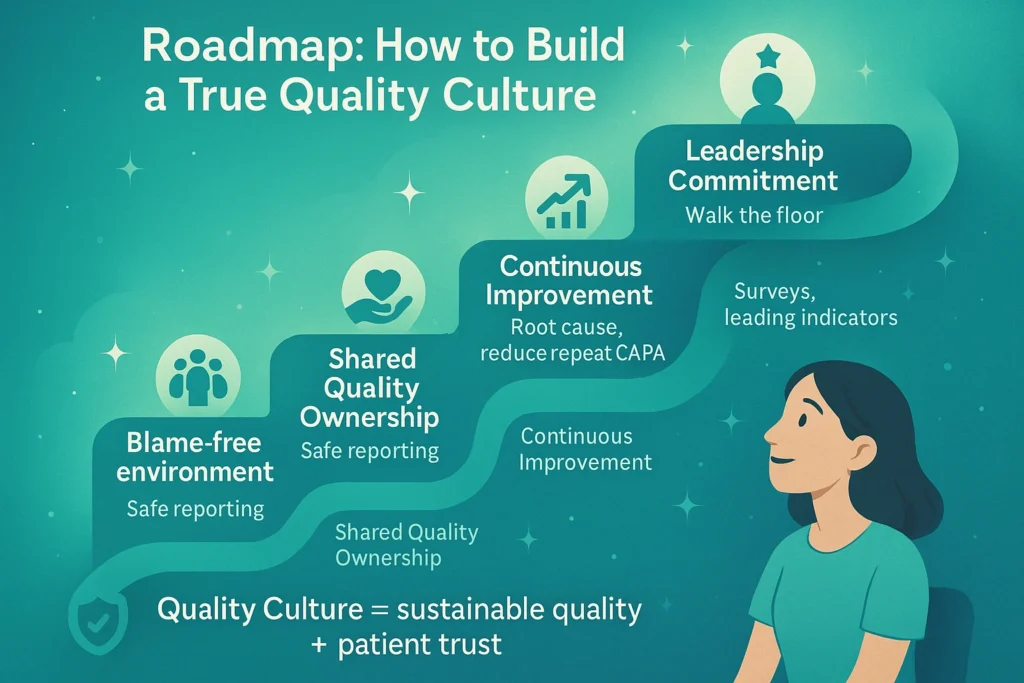
1. Leadership Commitment
- Leaders must model quality behavior: walking production floors, engaging in clinical operations reviews, and asking patient-focused questions.
- Communication should consistently link business goals to patient safety.
2. Empowered Employees
- Implement confidential or anonymous reporting systems.
- Recognize employees who identify near-misses, reframing “mistakes” as opportunities for learning.
3. Shared Ownership
- Embed quality KPIs across all departments.
- For example, clinical operations teams can track protocol deviation rates; manufacturing can track right-first-time batch performance.
4. Continuous Improvement
- Go beyond “human error” as a root cause.
- Apply systemic thinking: inadequate training, flawed procedures, or cultural disincentives often lie beneath.
5. Measuring Culture
- Use tools such as the PDA Quality Culture Assessment Model.
- Track leading indicators: employee surveys, deviation reporting trends, CAPA recurrence, and first-time-right performance.
Future Outlook: Culture as Competitive Advantage
The coming decade will see the industry adopt AI-driven analytics, decentralized clinical trials, and globalized supply chains. These innovations bring both opportunity and risk. Technology can improve compliance systems, but without a robust culture, it will not prevent failures.
Companies that embed culture will benefit from:
- Fewer regulatory observations.
- Faster approvals and greater regulator trust.
- Stronger employee retention and engagement.
- Enhanced reputation with patients, payers, and partners.
In a competitive global marketplace, quality culture is no longer optional — it is a differentiator.
Conclusion
Compliance builds systems; culture sustains them. In pharmaceuticals and clinical research, where patient lives are at stake, regulators are right to demand more than SOPs. A true quality culture is not measured in documentation, but in daily behaviours that prioritize safety, integrity, and continuous improvement.
Organizations that move beyond compliance to embrace culture will not only meet regulatory expectations but will build trust, resilience, and sustainable success.
References
- U.S. Food & Drug Administration (FDA). (2023). Inspection Observation Data (Form 483). Available at: https://www.fda.gov/inspections-compliance-enforcement-and-criminal-investigations/inspection-references/form-fda-483
- European Medicines Agency (EMA). (2008). ICH Q10: Pharmaceutical Quality System. Available at: https://www.ema.europa.eu/en/ich-q10-pharmaceutical-quality-system
- Medicines and Healthcare products Regulatory Agency (MHRA). (2021). GXP Data Integrity Guidance and Definitions. Available at: https://www.gov.uk/government/publications/gxp-data-integrity-guidance-and-definitions
- Parenteral Drug Association (PDA). (2022). Quality Culture Survey Report. Available at: https://www.pda.org/global-single-use-quality-culture-survey
- World Health Organization (WHO). (2020). Good Manufacturing Practices (GMP) for Pharmaceutical Products. Available at: https://www.who.int/publications/i/item/9789240010763