Introduction

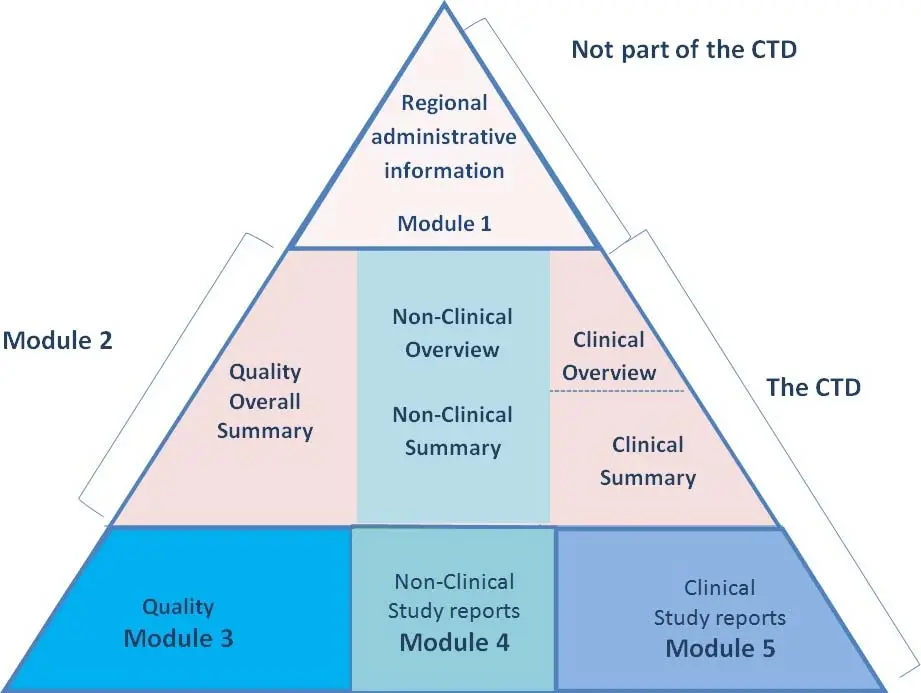
The Common Technical Document (CTD) is the internationally recognized framework for organizing pharmaceutical regulatory submissions. Developed by the International Council for Harmonization (ICH), it harmonizes documentation across regions by dividing content into five distinct modules.
CTD Module 1 is unique—it contains region-specific administrative and prescribing information, acting as the gateway to the harmonized Modules 2 to 5. Discover why this module is essential for successful global drug submissions. Understanding the contents, structure, and regional variations of Module 1 is necessary for submitting compliant applications to agencies such as the FDA (U.S.), EMA (EU), TGA (Australia), and others. Below, we explore the purposes, components, regional differences, key best practices, and tools necessary to master regulatory compliance in Module 1
What Is Module 1?
By design, Module 1—titled “Administrative Information and Prescribing Information”—houses documentation required by specific regulatory authorities that fall outside the harmonized content of Modules 2–5. It’s often said that Module 1 is “not truly part of the CTD” because of that regional specificity
The Common Technical Document format has revolutionized global regulatory review processes by allowing seamless cross‑region dossier reuse. However, Module 1 remains custom for each jurisdiction and must be tailored accordingly
Module 01 Structure (General Overview)
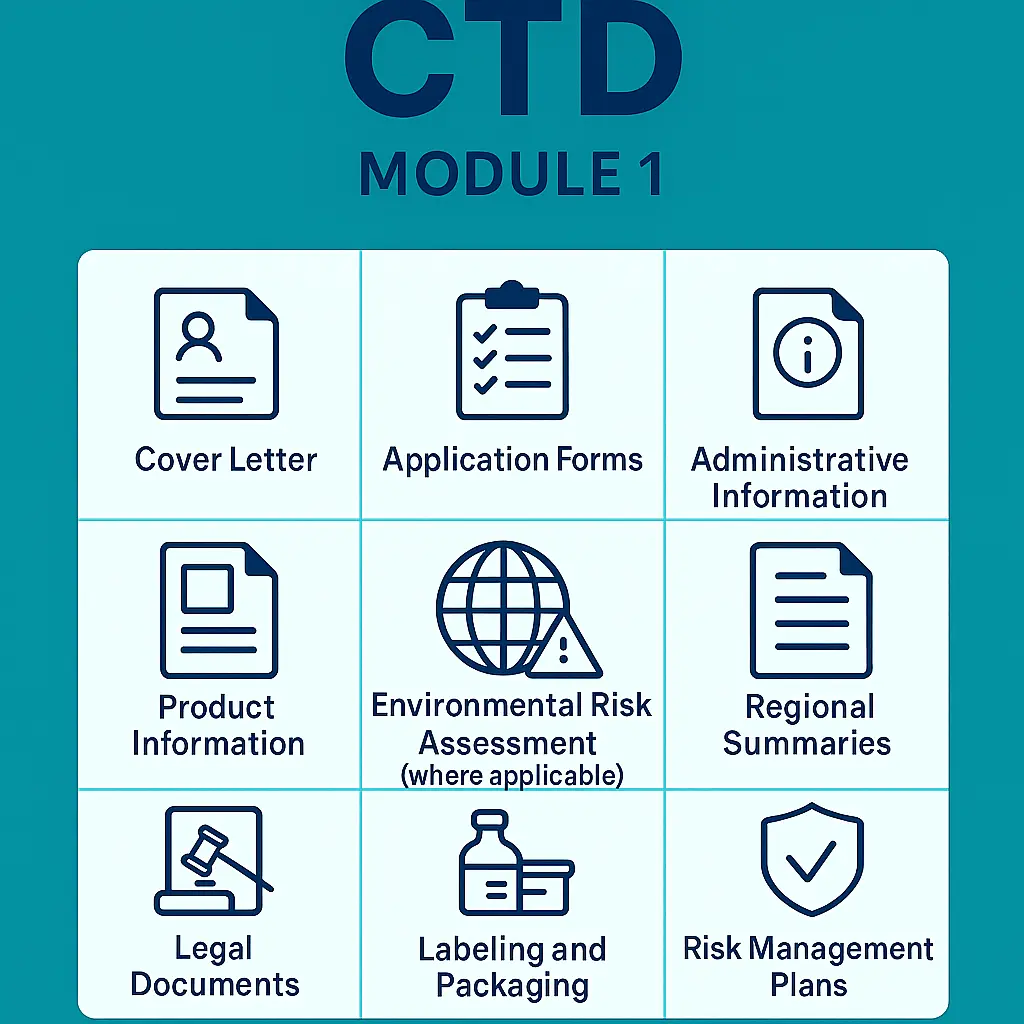
The structure of Module 01 varies by region, but typically includes the following sections:
- Cover Letter
- Application Forms
- Administrative Information
- Product Information
- Environmental Risk Assessment (where applicable)
- Regional Summaries
- Legal Documents
- Labeling and Packaging
- Risk Management Plans
Key Components of Module 01
1-Cover Letter
The cover letter serves as a concise summary of the submission and plays an essential role in framing the overall dossier. It typically includes the type of submission—such as NDA, ANDA, or MAA—a reference to any previous communications, a list of enclosed documents, and the signature of the responsible individual. The tone should remain professional and the content brief, as regulatory authorities value clarity and directness.
2-Application Forms
Each regulatory region mandates its own application forms. For example, the U.S. FDA requires Form 356h, the European Medicines Agency (EMA) uses the EU Application Form, and Australia’s Therapeutic Goods Administration (TGA) mandates the specific form for prescription medicine registration. It is vital that all forms are completed with accuracy, duly signed, and properly dated to avoid delays in review.
3-Administrative Information
This section contains core administrative data such as:
- the applicant’s details
- manufacturer information,
- Specifics about the drug, including its name, dosage form, and strength.
- It also includes the regulatory history and, if applicable, information about an orphan drug designation. Accuracy and completeness here help prevent initial validation issues.
4-Product Information
This portion of Module 01 includes crucial product-related documentation, such as:
- The Summary of Product Characteristics (SmPC)
- the package leaflet
- label mock-ups
- In the case of EU submissions: Braille text. Consistency in terminology, presentation, and formatting across all documents is critical to avoid questions or rejections from regulatory authorities.
5-Environmental Risk Assessment (ERA)
The ERA is required in the European Union and several other jurisdictions. It evaluates the potential environmental impact of the medicinal product. Failure to provide this assessment where required can lead to significant regulatory pushback.
6-Legal Documents
Legal documentation includes letters of authorization, patent details, debarment certifications, and declarations of compliance with acts such as the Pediatric Research Equity Act (PREA) in the U.S. It also encompasses GMP certifications and licenses for manufacturing sites. These documents help establish the legal and regulatory standing of both the applicant and the product.
7-Risk Management Plan (RMP)
Often a requirement for new chemical entities or high-risk medications, the Risk Management Plan outlines how the applicant intends to monitor, minimize, and manage potential risks associated with the medicinal product. The inclusion of a well-structured RMP can support a smoother evaluation process.
Module 01: Region-Specific Differences
Understanding the regional differences in Module 01 is essential for a compliant and successful submission. For the FDA in the United States, there is a strong emphasis on Form 356h, along with additional documents such as the User Fee Cover Sheet (Form FDA 3397), Field Copy Certification, and debarment certifications.
The EMA in the European Union requires product information to be submitted in all 24 official EU languages. It also demands a detailed Environmental Risk Assessment, a Pediatric Investigation Plan (PIP) compliance statement, and labeling that conforms to QRD templates.
In Japan, the Pharmaceuticals and Medical Devices Agency (PMDA) requires Japanese-language documents, translation certificates, and domestic GMP inspection reports.
Health Canada mandates the use of the Drug Submission Application Form (HC/SC 3011), compliance with product monograph requirements, and bilingual labeling in French and English.
Regional Variations in Module 01
Each country customizes Module 01 to meet its local regulatory needs. For example, Australia’s TGA explicitly defines Module 01 as a region-specific component that includes administrative documentation, prescribing information, and registration particulars. TGA’s guidance for OTC and prescription medicines outlines detailed sectioning, including the table of contents, administrative forms, expert declarations, and pharmacovigilance planning.
Health Canada frequently updates its Module 01 requirements to include pediatric development plans and foreign regulatory information. Items like “note to reviewer” sections, fee forms, and compliance notices reflect the evolving nature of Canadian regulatory expectations.
In global contexts such as WHO prequalification, Module 01 becomes critical for vaccine submissions, as it includes administrative information not duplicated elsewhere in the dossier. This information supports the harmonization of Modules 2–5 for global eligibility and approval.
In Bangladesh, the Directorate General of Drug Administration (DGDA) follows a similar structure to the ICH model but requires localized documents and labeling specific to national requirements.
Why Module 01 Matters
Module 01 is often referred to as the regulatory gatekeeper. It creates the first impression of a submission; thus, missing or incomplete administrative content can lead to delays or even outright rejection. Since this module is region-specific, it ensures that the application complies with the legal, linguistic, and structural standards of the target regulatory authority.
Additionally, Module 01 acts as a bridge between harmonized global data (Modules 2–5) and local regulatory expectations. It plays a vital role in submission validation and tracking, especially in the electronic Common Technical Document format.
It houses essential metadata and identifiers used by regulatory validation tools. With the rollout of the Electronic Common Technical Document, the importance of Module 01 increases further as it now includes advanced metadata, enhanced document life-cycle tracking, and improved content tagging—all of which are vital for submission accuracy and agency interaction.
Best Practices for Crafting Module 01
Several best practices can help ensure the success of a Module 01 submission. First, always use the correct regional templates and ensure you’re downloading the latest versions from official regulatory websites, as these forms change frequently.
Maintain a detailed table of contents under Section 1.1, which should include all dossier components along with document-level details and cross-links, particularly important in electronic submissions. Consistency is key—ensure that product names, dosages, and formulation details in Module 01 perfectly align with those presented in Modules 2 through 5.
Adherence to each agency’s file naming conventions is critical, especially in electronic submissions, as improper naming can trigger validation errors. It’s also crucial to complete all mandatory sections; missing pharmacovigilance statements, pediatric declarations, or master file references can mark the submission as incomplete. Prior to submission, use available validation tools or checklists provided by the regulatory authorities to identify and resolve potential errors early.
Lastly, stay informed about regulatory updates, such as Canada’s evolving pediatric content requirements or the global shift to eCTD features, to remain compliant with current expectations.
Tools to Support Module 01 Preparation
Modern regulatory software platforms greatly simplify the creation and management of Module 01. Tools like Lorenz DocuBridge, EXTEDO eCTDmanager, Phlexglobal PhlexSubmission, GlobalSubmit, and Veeva Vault RIM provide end-to-end support, including folder structuring, document version control, form completion, and built-in compliance checks with global electronic common technical document validation rules.
These platforms are particularly useful in managing multiple regional versions of Module 01 across global submissions, ensuring harmonization while maintaining local specificity.
Real-World Insight from Regulatory Professionals
Feedback from regulatory professionals provides valuable context for the importance of Module 01. One experienced user on Reddit highlighted that it can be thought of not just as a content standard but also as a structured folder system governed by metadata and content rules.
The eCTD comprises the directory structure, the XML backbone, and the actual document files. Variability in Module 01 between regions is a consistent challenge, as each agency sets its own requirements and validation criteria. These real-world experiences stress the need for careful planning and understanding of both the technical and administrative components involved.
Navigating the Future: eCTD and Beyond
The regulatory landscape is evolving with the global implementation of the eCTD version, first adopted by the FDA in late 2022. This update introduces modernized metadata handling, improved lifecycle management, and two-way communication capabilities between applicants and regulatory authorities.
Module 01 becomes even more central under eCTD, as it is now deeply integrated with the metadata and validation systems that support regulatory workflows. The future of submissions will likely involve greater traceability, real-time feedback, and enhanced automation in document preparation. A well-prepared and compliant Module 01 will be the foundation for a smoother and more efficient submission life-cycle in this new era.
Conclusion
Module 01 is a critical component of any global regulatory submission. As the region-specific gateway to the full dossier, it must be carefully crafted to meet local requirements while supporting the harmonized content in Modules 2 through 5.
A strong Module 01 ensures regulatory compliance, reduces the risk of delays, and increases the likelihood of first-pass acceptance. With the advent of eCTD and the growing emphasis on metadata and automation, having a robust and flexible Module 01 strategy is essential for navigating today’s complex regulatory environment. Mastery of this module not only strengthens the submission but also supports faster review and approval timelines across diverse regulatory jurisdictions.
References
LinkedIn. (2023). DGDA adopts the Common Technical Document (CTD) format for registration. https://www.linkedin.com/pulse/dgda-adopts-common-technical-document-format-registration-tareq-
International Council for Harmonization (ICH). (n.d.). The Common Technical Document (CTD). https://www.ich.org/page/ctd
Australian Government Department of Health and Aged Care – Therapeutic Goods Administration (TGA). (2022). Common Technical Document (CTD) for OTC medicines.https://www.tga.gov.au/resources/resource/guidance/common-technical-document-module-1-otc-medicines
Australian Government Department of Health and Aged Care – Therapeutic Goods Administration (TGA). (n.d.). CTD Module 1: Administrative and product information. https://www.tga.gov.au/resources/resource/reference-material/ctd-module-1
Health Canada. (2021). Organization and document placement for the Canadian Module 1. Government of Canada. https://www.canada.ca/en/health-canada/services/drugs-health-products/drug-products/applications-submissions/guidance-documents/organization-document-placement-canadian-module-1.html
World Health Organization (WHO). (2020). Preparation and submission of CTD for vaccines: WHO prequalification. https://extranet.who.int/prequal/vaccines/ctd-preparation-submission
Pubrica. (n.d.). CTD Structure Overview Modules 1 to 5. https://pubrica.com/services/medical-writing/regulatory-writing/ctd-structure-overview-modules/
Pharmacy InfoLine. (n.d.). Common Technical Document (CTD). https://pharmacyinfoline.com/common-technical-document-ctd/
Pharmaceutical Carrier. (2022). CTD guidance: ICH CTD or eCTD? https://pharmaceuticalcarrier.com/index.php/2022/07/27/common-technical-document-ctd-guidance-ich-ctd-or-ectd/
Scribd. (n.d.). CTD and eCTD explained. https://www.scribd.com/document/437149162/CTD-and-eCTD
Reddit. (2023). Discussion: Differences in CTD and eCTD submission across regions [Discussion forum post]. Reddit. https://www.reddit.com/r/RegulatoryClinWriting/comments/187weax
Reddit. (2023). eCTD v4.0 adoption and metadata insights [Discussion forum post]. Reddit. https://www.reddit.com/r/RegulatoryClinWriting/comments/11qyago


