Artificial Intelligence (AI) and Machine Learning (ML) are revolutionizing drug development, offering transformative capabilities across the spectrum of pharmaceutical research. By integrating AI-driven computational models with insights from experimental (wet lab) investigations, the field of drug delivery has evolved to prioritize precision, efficiency, and patient-specific therapies. This technical commentary delves into the dual roles of wet lab experimentation and computational innovations, emphasizing how nanoparticle-based systems, predictive analytics, and real-time monitoring are redefining therapeutic strategies. Additionally, this article critiques the ethical, regulatory, and technical challenges inherent in this paradigm shift while proposing collaborative pathways for future advancements.1
Bridging Wet Lab Innovation: AI-Driven Drug Delivery Systems as Nanoparticle Engineering
The pharmaceutical industry faces persistent challenges in enhancing the efficacy, safety, and personalization of therapies. Traditional methods, which often rely on trial-and-error approaches, are time-intensive and cost-prohibitive. AI and ML offer solutions by enabling data-driven insights, predictive modeling, and optimization of drug delivery systems. This convergence of wet lab research with computational methodologies is pivotal in achieving breakthroughs in precision medicine. AI’s role in drug development encompasses various stages, including target identification, hit-to-lead optimization, and clinical trial design. AI integrates with nanotechnology to engineer smart drug delivery systems that respond to specific physiological triggers, enhancing bioavailability and minimizing systemic exposure.
The pharmaceutical landscape is at a critical juncture, with AI and ML poised to address longstanding inefficiencies in drug discovery, formulation, and delivery. Traditional methods, which often rely on resource-intensive and time-consuming processes, struggle to keep pace with the complex demands of modern therapeutics, especially in the era of personalized medicine. AI’s capacity to analyze vast datasets, identify molecular patterns, and predict therapeutic outcomes has catalyzed a fundamental shift in drug development. Notably, AI’s integration with nanotechnology has unlocked new possibilities in designing drug delivery systems that optimize therapeutic outcomes while minimizing adverse effects.1,2
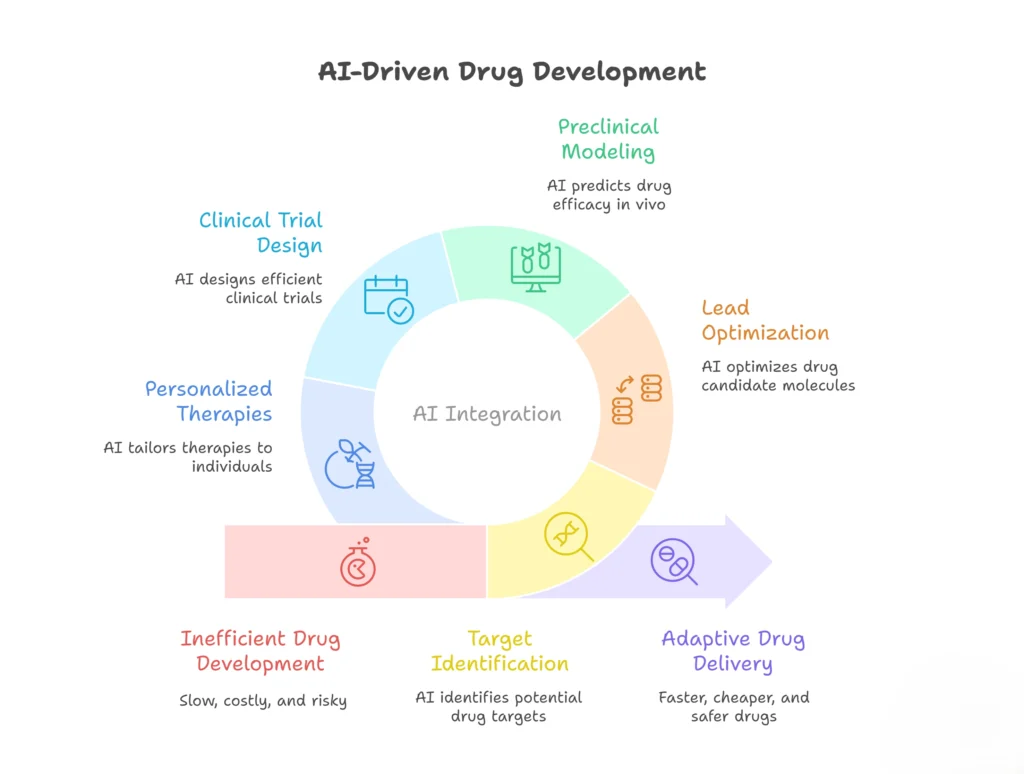
It is emphasized that AI’s role extends beyond target identification to encompass the entire drug development pipeline, including lead optimization, preclinical modeling, and clinical trial design. Meanwhile, it is underscored AI’s role in personalizing therapies through patient-specific data integration, thereby improving dosing accuracy and reducing systemic risks. The intersection of wet lab research and computational insights forms the bedrock of these innovations, fostering a more adaptive and efficient approach to drug delivery.2,3
Nanoparticles and Smart Drug Delivery Systems1,2,4
Wet lab research has played a pivotal role in advancing nanoparticle-based drug delivery systems, which offer precise targeting and controlled release mechanisms. The ability to engineer nanoparticles that respond to specific physiological cues, such as pH variations or enzymatic activity, represents a significant leap in therapeutic precision. Jena et al. discuss how AI-driven models optimize the size, shape, and surface characteristics of nanoparticles to ensure targeted drug delivery, enhancing both bioavailability and patient outcomes. This tailoring of drug carriers not only improves therapeutic efficacy but also reduces off-target effects, a critical consideration in minimizing toxicity and adverse reactions.
Furthermore, controlled release systems are integral to maintaining optimal drug concentrations over extended periods. Wet lab experiments have demonstrated the feasibility of designing polymers and hydrogels that modulate drug release in response to external stimuli. AI augments this process by analyzing pharmacokinetic and pharmacodynamic data, enabling the prediction and customization of release profiles tailored to individual patient needs. This synergy between computational predictions and experimental validation underscores the importance of an integrated approach to drug delivery innovation.
AI Models, Predictive Analytics, and Virtual Simulations2
On the computational front, AI models have become indispensable tools in predicting molecular interactions, optimizing lead compounds, and assessing drug-likeness. Gholap et al. highlight the use of advanced neural networks and deep learning algorithms to analyze vast chemical and biological datasets. These models facilitate the identification of promising drug candidates by predicting binding affinities, toxicity profiles, and ADME properties, thus streamlining the drug discovery process. Unlike traditional methods that rely heavily on empirical testing, AI-driven approaches offer a data-centric alternative that accelerates discovery timelines while reducing costs. Machine learning algorithms also play a crucial role in adaptive drug delivery systems.

By continuously monitoring patient data and environmental conditions, AI can dynamically adjust drug dosing and release patterns to achieve optimal therapeutic outcomes. This real-time feedback loop, facilitated by wearable sensors and IoT devices, represents a paradigm shift in personalized medicine. Moreover, virtual clinical trials, supported by in silico modeling, enable the simulation of drug responses across diverse patient populations, reducing the reliance on costly and time-intensive physical trials.
However, these computational advancements are not without limitations. The accuracy of AI models is contingent upon the quality and diversity of training datasets. Biases in data collection can lead to skewed predictions, necessitating robust data curation and validation protocols. Additionally, the interpretability of AI models remains a significant challenge, particularly in regulatory contexts where transparency and accountability are paramount.
Ethical and Regulatory Challenges: Navigating Complexity and Accountability
The integration of AI in drug delivery raises complex ethical and regulatory issues that must be addressed to ensure patient safety and public trust. One of the foremost concerns is data privacy, especially given the sensitive nature of patient information used to train AI models. Ensuring compliance with data protection regulations, such as the General Data Protection Regulation (GDPR), is essential to mitigate risks associated with data breaches and unauthorized access.
The “black-box” nature of many AI models further complicates regulatory approval processes. Regulators require clear explanations of how AI algorithms generate predictions, yet the complexity of deep learning models often hinders interpretability. Developing methods for model explainability and transparency is crucial for fostering trust and facilitating regulatory compliance. Collaborative efforts between technologists, clinicians, and policymakers will be essential in creating a framework that balances innovation with ethical accountability.
Additionally, the ethical implications of AI-driven decision-making in clinical settings cannot be overlooked. While AI can enhance diagnostic accuracy and treatment planning, it must be used as a complementary tool rather than a replacement for clinical judgment. Establishing guidelines for AI-assisted decision-making will be critical in ensuring that human oversight remains central to patient care.
Future Perspectives: Convergence of AI with Emerging Technologies2
The future of AI-driven drug delivery lies in its convergence with other cutting-edge technologies. CRISPR-based gene editing, blockchain for secure data management, and IoT-enabled devices for real-time patient monitoring are poised to enhance the precision and adaptability of drug delivery systems. These synergistic technologies have the potential to create highly individualized therapies that respond dynamically to patient needs.
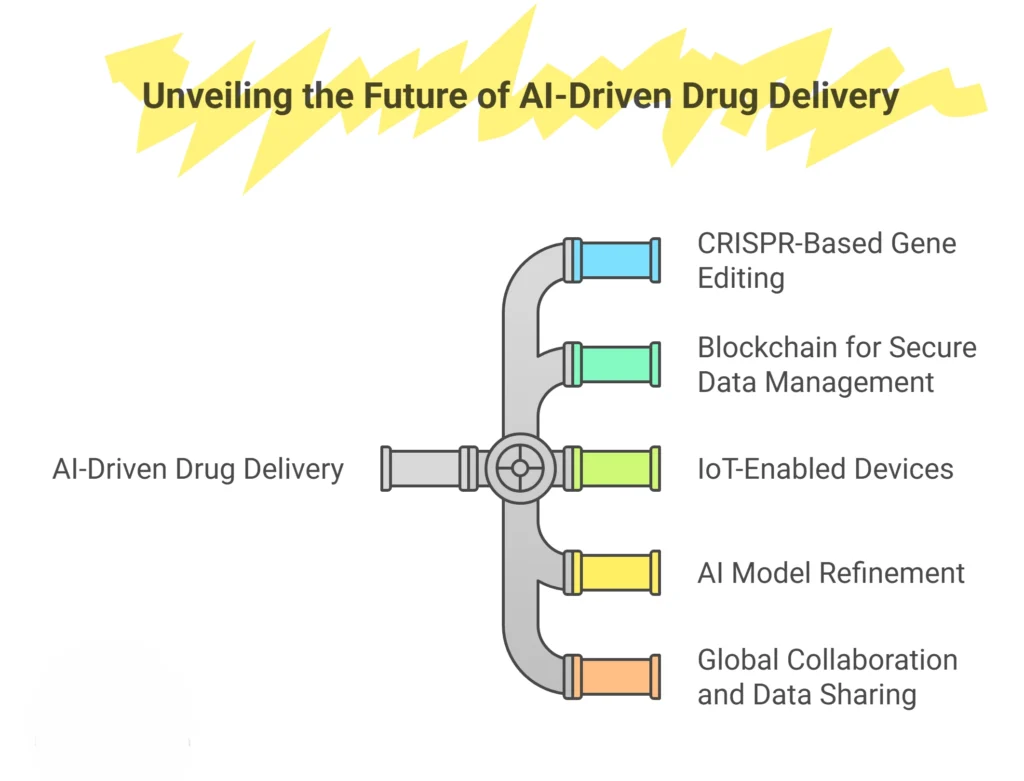
Moreover, ongoing research should focus on refining AI models to improve their accuracy, generalizability, and interpretability. Addressing biases in training data and developing robust validation frameworks will be essential for advancing AI’s role in drug delivery. Global collaboration and data sharing will also play a pivotal role in accelerating innovation and ensuring equitable access to advanced therapies.
Conclusion
AI and ML are redefining the paradigm of drug development and delivery, offering unprecedented opportunities to enhance precision, efficiency, and patient outcomes. By integrating computational insights with experimental validation, the pharmaceutical industry can achieve transformative breakthroughs. However, realizing this potential will require addressing ethical and regulatory challenges, fostering interdisciplinary collaboration, and advancing AI technologies to meet the complex needs of modern medicine. The future of AI-driven drug delivery is bright, but its success hinges on the collective efforts of researchers, technologists, clinicians, and policymakers.
References
