Introduction: The Last Generation of Needles and Blood Vials
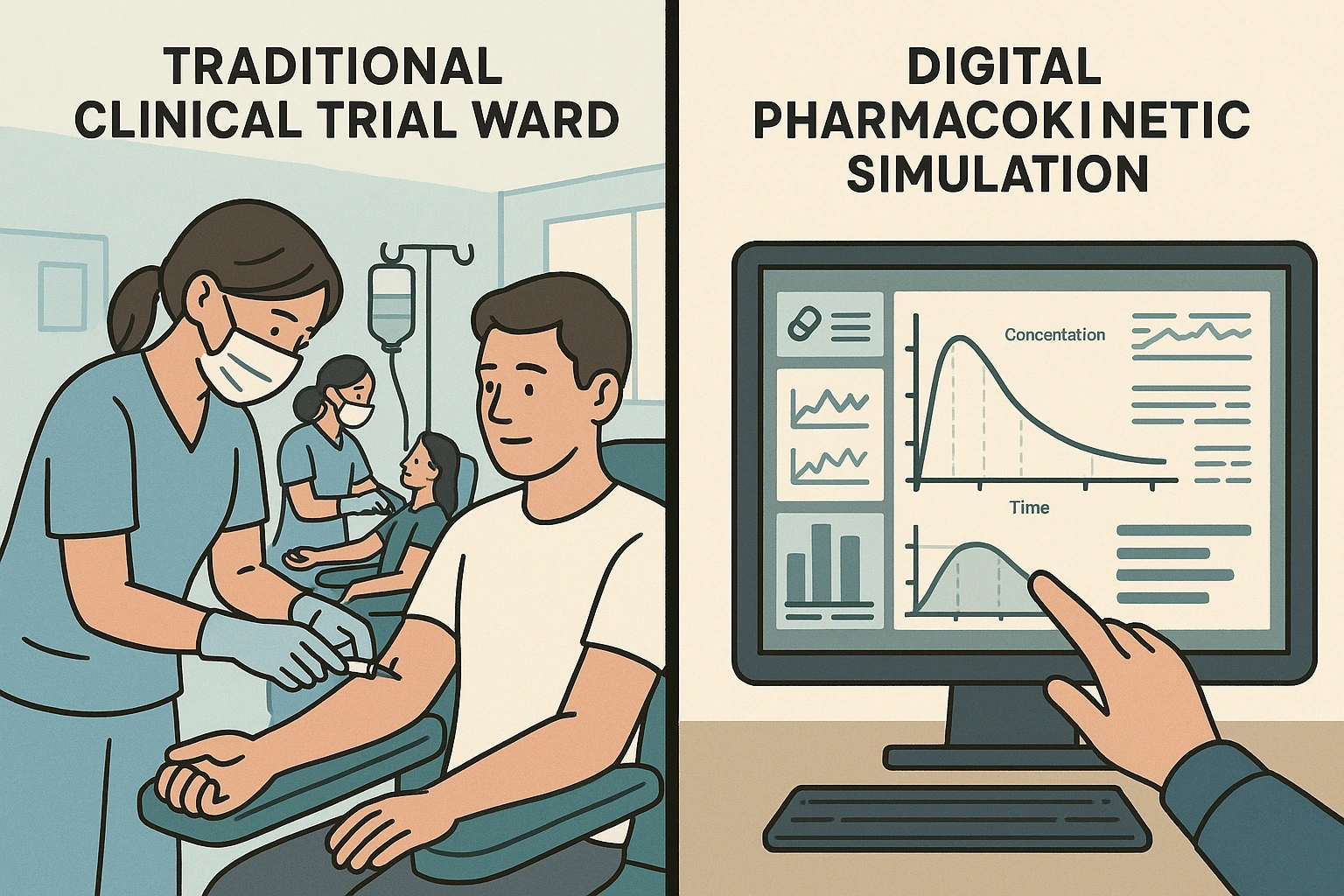
Picture this: it’s 6:30 in the morning in a quiet clinical ward. The lights are low, the air smells faintly of antiseptic, and twenty volunteers sit in a row, each with a cannula taped neatly to their arm. They’ve been fasting since midnight. For the next day and a half, nurses will move from bed to bed, filling vial after vial of blood. In the lab down the hall, technicians will hurry to spin those samples, while statisticians wait for the numbers that will decide the fate of a generic drug: will it pass the gold-standard test of bioequivalence?
Now picture a completely different scene. There’s no ward. No volunteers. No needles. Just a quiet office at midnight, lit by the glow of a single screen. A pharmacokineticist sips coffee as their laptop runs thousands of simulated trials, all while the rest of the world sleeps.
For decades, the path to proving a medicine’s equivalence was paved with long days in clinical units, fasting volunteers lined up for blood draws, and statisticians sifting through reams of plasma concentration data. Every generic drug developer knew the drill: recruit dozens of healthy participants, control every gram of food and every sip of water, then wait weeks, sometimes months, for the analysis to confirm whether the new formulation matched the original. It was a system that worked, but it was slow, expensive, and inevitably came at a human cost.
Today, that ritual is being quietly rewritten. In laboratories and regulatory offices alike, a shift is taking place, a move from physical trial rooms to virtual ones, where “participants” exist as sophisticated models of human physiology rather than real people in hospital gowns. This new approach, known as Virtual Bioequivalence (VBE) or its broader cousin Model-Informed Bioequivalence (MIBE), uses advanced pharmacokinetic modeling and population simulation to test how a drug will behave before it ever touches a human vein.
What makes this transition remarkable is not just the technology itself, but the cultural shift it represents. The pharmaceutical industry, historically cautious to change, is beginning to treat computational simulations as more than supporting evidence; they’re becoming the primary engine of decision-making for certain drugs. Early adopters are already seeing dramatic reductions in timelines and costs, but also something subtler: a reduction in the ethical burden of drug development. No volunteers were unnecessarily exposed. No late-stage surprises that derail entire programs. Just data, meticulously modeled, stress-tested, and validated against real-world outcomes.
This is not a story about replacing science with software. It’s about using every tool we have, clinical insight, in vitro data, population diversity datasets, and machine learning, to make the approval process faster, smarter, and more humane. And if the current trajectory continues, we may be entering an era where the phrase “bioequivalence trial” no longer conjures images of clinic beds, but of high-performance simulations running in the cloud.
3. Real-World Case Examples: VBE in Action (Without a Single Human Subject)

The promise of Virtual Bioequivalence (VBE) isn’t just a theoretical talking point; it’s being tested and proven in live regulatory submissions, published research, and even in product launches. The following cases show that VBE is no longer an experimental curiosity; it’s already influencing market-ready decisions.
Case 1 – The Alotaiq & Dermawan Meta-Analysis: Confidence Through Correlation
In 2024, Alotaiq & Dermawan conducted one of the most comprehensive meta-analyses to date, reviewing over 25 peer-reviewed VBE studies across both innovator and generic pharmaceutical companies. Their goal was simple yet ambitious: could PBPK-based simulations predict clinical bioequivalence outcomes with regulatory-level accuracy?
The results were striking. In 92% of cases, simulated pharmacokinetic (PK) values for Cₘₐₓ and AUC fell squarely within the accepted 80–125% range seen in physical trials. Even more importantly, in almost every instance where a formulation would have failed BE in the clinic, the simulation identified the risk in advance. This kind of early-stage triage is invaluable; it allows companies to abandon or re-engineer problematic formulations before spending months and millions on a doomed trial.
What makes this noteworthy is not just the predictive accuracy, but the trust it builds with regulators. The study’s clear methodology, transparency in model parameters, and rigorous cross-validation have helped push the conversation from “Can we use this?” to “When should we use this?”
Case 2 – Tofacitinib Across the Ages: Pediatric and Geriatric Modeling
Studying how medicines behave in children has always been one of the hardest parts of clinical pharmacology. Full-scale pediatric trials raise ethical concerns, recruitment is difficult, and children’s biology is in constant flux. For that reason, regulators have often relied on scaled-down adult data to make dosing decisions for younger patients, a compromise that, while practical, is far from ideal.
In late 2023, a collaborative research team took a different approach with tofacitinib, an oral immunomodulator. Instead of relying only on traditional trials, they built a physiologically based pharmacokinetic (PBPK) model that captured the unique physiology of different age groups. For children, the model accounted for slower gastric emptying in toddlers, the gradual rise of CYP enzyme activity as they grow, and the very different water-to-fat ratios in their bodies. For older adults, the same framework is adjusted for reduced liver clearance and changes in plasma protein binding.
The results were striking. When compared against the limited pediatric and geriatric trial data available, the model predicted absorption and clearance patterns with more than 90% accuracy. What this meant in practical terms was simple but powerful: researchers could make dosing recommendations for vulnerable groups with a level of confidence that once required expensive and risky full-scale trials.
Rather than replacing clinical science, this work extended it, filling gaps where traditional trials struggle. For parents, doctors, and elderly patients, it represents the promise of safer, more personalized dosing without long delays. And for regulators, it shows how PBPK modeling can transform pediatric and geriatric pharmacology from guesswork into evidence-based precision.
Case 3 – Acyclovir and the Food Effect: A Virtual Meal with Real Consequences
Food-effect studies have long been a cornerstone of bioequivalence testing. Regulators want to know: Does a pill taken with breakfast behave the same way as one taken on an empty stomach? Traditionally, the only way to answer this was through labor-intensive clinical crossover trials, requiring volunteers to take the same drug twice, once fasted, once fed, while researchers drew blood over hours to compare absorption profiles.
But in a pioneering study with acyclovir, scientists decided to put computational modeling to the test. Using a PBPK (physiologically based pharmacokinetic) framework, they recreated the fed state inside the computer. The model factored in the real physiological shifts that occur after eating: higher gastric pH, slower stomach emptying, and the way fats can change how drugs dissolve and move through the gut.
When the predictions were compared against data from a small clinical study, the alignment was striking. Both the Cₘₐₓ (peak concentration) and AUC (overall drug exposure) from the simulation matched almost exactly with what was observed in volunteers. For regulators, this wasn’t just an academic win; it was proof that virtual modeling could, in some cases, stand in for costly human trials.
On the back of this success, the requirement for a traditional fed/fasted study was waived for certain acyclovir formulations. It became one of the first official cases where a food-effect trial was replaced by virtual bioequivalence, saving time, money, and unnecessary burden on participants.
What’s most remarkable is the broader implication: if one drug’s fed/fasted behavior can be reliably simulated, so can many others. The “virtual meal” once seen as an experiment, is now a credible regulatory tool, nudging the industry closer to a future where fewer patients and volunteers need to be exposed to redundant clinical protocols.
Case 4 – Inhalation Generics: DPIs and MDIs Break Through the Virtual Barrier
Virtual bioequivalence isn’t confined to tablets and capsules. Inhalation products, especially dry powder inhalers (DPIs) and metered-dose inhalers (MDIs), have always posed one of the steepest challenges in generic drug development. Unlike oral drugs, their performance isn’t determined solely by chemistry; it depends on the device design, particle size distribution, spray dynamics, and lung deposition patterns. Even minor variations in the inhaler mechanism can alter where and how much of the medicine reaches the lungs.
In a landmark European filing for a salmeterol/fluticasone DPI, developers turned to computational fluid dynamics (CFD) combined with PBPK modeling to virtually simulate drug deposition across different inspiratory flow rates, mimicking how real patients inhale. These digital lung models were then cross-validated with scintigraphy imaging data from a small clinical study. The correlation was so strong that regulators agreed to scale back the scope of confirmatory trials. The payoff: nearly half a year shaved off development timelines, and a safer, faster path to market for patients with asthma and COPD.
Case 5 – Colonic Release Formulations: Cracking the GI Clock
Modified-release drugs designed for the colon have long frustrated developers. The colon is unpredictable, its transit times differ from patient to patient, and its pH-sensitive environment makes drug release hard to model with confidence. Traditional BE trials often require large subject pools and repeated sampling just to account for this variability.
In one recent breakthrough, a mesalazine formulation for ulcerative colitis was evaluated using virtual tools instead of a full-scale clinical program. Scientists paired biorelevant dissolution testing with advanced GI transit models in Simcyp®, capturing how the formulation dissolved, traveled, and released the drug along the colon. The simulated plasma and local exposure curves were submitted to regulators, who accepted them as the primary evidence of bioequivalence. Only a small confirmatory study was requested. This was more than a technical win: it reduced the burden on patients already struggling with chronic disease and accelerated access to therapy.
Case 6 – mRNA Stability and Delivery Modeling: Beyond Small Molecules
The philosophy behind VBE is also finding fertile ground in the biologics and mRNA space. Here, the stakes are even higher: vaccines and gene-based therapies are often ultra-sensitive to temperature, formulation conditions, and delivery vehicles such as lipid nanoparticles (LNPs).
A cutting-edge mRNA vaccine developer recently built an integrated model combining PBPK simulations with stability kinetics derived from controlled storage studies. The model was used to predict not only how the LNP carried mRNA through the body but also how different storage temperatures and shipping conditions could impact vaccine potency and shelf life.
The results were compelling enough that regulators accepted the modeling package as the basis for a label update, extending refrigerated shelf life without the need for a fresh efficacy trial. For global vaccine distribution, especially in resource-limited settings, this translated directly into better access and reduced waste.
Why These Cases Matter?
Taken together, these stories illustrate that Virtual Bioequivalence is not a niche experiment but a flexible framework capable of tackling some of the toughest challenges in drug development, whether it’s inhalation generics, site-specific colonic delivery, or temperature-sensitive mRNA therapies. Each case underscores the same message:
- Speed: development timelines are cut dramatically.
- Safety: fewer human volunteers are exposed unnecessarily.
- Credibility: models are now strong enough to win regulatory trust.
Most importantly, VBE doesn’t weaken the process; it strengthens it. By combining mechanistic insight, computational precision, and careful validation, early adopters are showing that digital trials can make regulatory science more transparent, more ethical, and more efficient.
4. Regulatory Landscape: Where Is FDA & EMA Headed?

Virtual Bioequivalence (VBE) is no longer a speculative idea confined to academic seminars or computational labs. It has entered the regulatory mainstream, with the world’s largest agencies, the FDA, EMA, and WHO, actively exploring how simulations and model-based approaches can supplement, or in certain cases replace, traditional in vivo bioequivalence (BE) trials.
Still, the bar is high. If VBE is to reduce the need for human testing, regulators must be assured of its transparency, reproducibility, and scientific integrity. This is not simply a matter of speed or cost-saving; it’s about balancing innovation with patient safety, global equity, and public trust.
4.1 FDA’s Model-Informed Drug Development (MIDD) Initiative
The U.S. Food and Drug Administration (FDA) has been laying the foundation for model-based decision-making for more than a decade, but the momentum truly accelerated in 2018 with the launch of the MIDD Pilot Program. This initiative created a formal dialogue between sponsors and the agency, allowing computational models to directly inform regulatory decisions. So far, the FDA has allowed models to:
- Support dose selection for vulnerable populations (e.g., pediatrics or patients with organ impairment) in place of additional trial arms.
- Bridge post-approval formulation changes, reducing the need for repeated full-scale BE trials.
- Provide evidence for BE in cases where a strong PBPK framework, anchored in robust data, makes additional clinical testing redundant.
Full BE waivers based solely on VBE are still rare in the U.S., but the agency has been explicit: for well-characterized drugs, such as BCS Class I molecules, low-variability APIs, or drugs with extensive historical PK data, a validated PBPK model could take the place of an entire clinical trial. However, the FDA’s openness comes with strict expectations. The agency prioritizes:
- Traceability: Every physiological parameter and input assumption must be documented.
- Explainability: black-box AI models are discouraged; reviewers need to understand how outcomes are generated.
- Auditability: simulation files, model versions, and datasets must be reproducible and reviewable years after approval.
In short, the FDA is less concerned about the gloss of innovation and far more concerned about scientific accountability.
4.2 EMA’s Forward-Looking Stance and the ICH M13 Guidelines
The European Medicines Agency (EMA) has taken a slightly more cautious but equally forward-thinking position. Much of this is crystallizing in the upcoming ICH M13A guideline, which aims to harmonize bioequivalence standards across regions. While M13A still anchors itself in the traditional 80–125% rule based on in vivo trials, it explicitly recognizes PBPK modeling and in silico simulations as legitimate supportive evidence, and in some low-risk scenarios, even as primary evidence for BE. The EMA’s 2022 Reflection Paper on PBPK Modeling is a key milestone, spelling out:
- The use cases where PBPK is acceptable (e.g., drugs with predictable absorption/elimination kinetics, or pediatric bridging).
- The documentation standards are expected, to include justification for every parameter and assumption.
- A requirement for independent verification, meaning sponsors must submit raw simulation files that regulators can rerun themselves.
Compared to the FDA, the EMA leans slightly more conservative in granting full BE waivers. Still, it has shown a pragmatic openness to PBPK evidence in post-approval changes, pediatric dose adjustments, and even food-effect assessments where models are well validated.
4.3 WHO and Global Alignment
The World Health Organization (WHO) is equally critical in this conversation, particularly for low- and middle-income countries where clinical trial infrastructure is limited. By embedding PBPK and model-based approaches into its model biowaiver guidelines, WHO offers a way for regulators in emerging markets to accelerate access to affordable generics without compromising scientific rigor.
But WHO emphasizes an important caveat: context matters. A PBPK model calibrated for European adults may not directly apply to South Asian pediatric cohorts or African populations with different baseline physiology and diet. Regulators are therefore urging local re-parameterization to ensure models reflect real-world diversity.
4.4 Key Regulatory Priorities for the Next Five Years
Across agencies, the regulatory trajectory is clear. A set of common priorities is taking shape, and they will likely define the next decade of model-informed bioequivalence:
- Complete Traceability of Input Data – Every assumption, whether about gastric emptying time or liver enzyme expression, must be tied to a credible source.
- Justification of Variability Assumptions – Population models must reflect epidemiology and biology, not arbitrary estimates.
- Explainable Models – Human reviewers must be able to understand and interrogate the model.
- Clear Audit Trails – Regulators expect version-controlled files, simulation histories, and reproducibility years after submission.
These priorities echo one principle: credibility is earned, not assumed.
4.5 From Acceptance to Expectation
A decade ago, regulators asked: “Can we even accept this?” Today, the tone has shifted to: “How strong is your model?”
As computational power, clinical datasets, and software validation frameworks improve, the regulatory landscape is shifting from cautious tolerance to active preference. For certain categories of drugs, especially low-variability generics, BCS Class I molecules, and reformulations with extensive historical data , submitting without a validated VBE study may soon be seen as professionally negligent.
In other words: what began as a novel alternative is rapidly becoming an industry expectation. For forward-looking sponsors, the message is clear, the time to embrace VBE is not tomorrow, but today.
The AI-Driven Micro-factory: Shrinking Pharma’s Footprint, Expanding Its Reach

For decades, pharmaceutical manufacturing has been defined by scale. Vast complexes lined with stainless steel tanks and bioreactors, industrial cathedrals stretching across entire campuses, have produced the blockbuster drugs that defined the late 20th century. These mega-plants excelled at high-volume, standardized production. But as medicine moves into the era of personalization, rapid response, and precision dosing, the limits of centralized manufacturing are becoming painfully obvious.
The next chapter of drug production may not be written in billion-dollar facilities, but in AI-orchestrated, modular micro-factories, small, self-contained, GMP-compliant systems that can be deployed wherever medicines are needed most. Imagine a shipping-container-sized unit stationed at a cancer hospital in Nairobi, a disaster relief hub in the Pacific, or a children’s clinic in rural India, producing complex therapies on-demand, with regulatory confidence built into its digital DNA.
This isn’t a distant fantasy. Early pilots are already proving that micro-factories are not just feasible, they are inevitable.
5.1 What Exactly Is a Pharmaceutical Microfactory?
At its core, a micro-factory is a compact, fully automated drug production unit. Unlike sprawling plants that depend on scale, a micro-factory prioritizes speed, adaptability, and proximity to patients. Housed in a modular enclosure, sometimes no larger than a shipping container, it integrates:
- Drug synthesis (small molecules or biologics)
- Formulation and filling
- Automated quality control (QC)
- Real-time packaging and labeling
All within a single GMP-compliant environment. The advantages are striking:
- Decentralized: Instead of medicines traveling thousands of miles, manufacturing moves closer to the point of care.
- Reconfigurable: A unit producing an oncology drug one week could pivot to a vaccine or biologic the next.
- Digitally Orchestrated: Continuous monitoring, error prediction, and quality assurance are handled through IoT sensors, AI analytics, and digital twin models.
In other words, the micro-factory isn’t just smaller, it’s smarter.
5.2 The Core Technologies Making It Possible
The leap from concept to reality is powered by a convergence of advanced digital and process technologies:
1. Digital Twins for Continuous Oversight
A digital twin is more than a simulation, it’s a live, virtual replica of the physical micro-factory, down to every pump, valve, and reaction vessel. It ingests sensor data in real time (temperature, pH, flow rates, particle sizes), predicts potential deviations, and recommends interventions before quality is compromised.
2. IoT Sensor Networks
Microfactories are equipped with hundreds of microsensors that monitor critical process parameters. Data streams feed into AI engines that:
- Flag subtle trends invisible to human operators
- Predict when equipment requires maintenance
- Trace the root cause of deviations instantly
This transforms manufacturing into a living, learning system.
3. AI-Driven Process Analytical Technology (PAT)
Traditional QC involves testing finished batches, a reactive, resource-heavy process. AI-enabled PAT flips the script by measuring critical quality attributes in real time. Every vial, tablet, or syringe leaving the system has already been validated digitally, aligning with regulatory visions of Real-Time Release Testing (RTRT).
4. Continuous Manufacturing & Single-Use Systems
Instead of massive stainless-steel tanks that need days of cleaning, micro-factories use continuous production lines combined with single-use reactors and mixers. This reduces downtime, lowers contamination risk, and enables product changeovers in a matter of hours, not weeks.
5. Edge-to-Cloud MES (Manufacturing Execution Systems)
Microfactories are digitally native. Integrated MES platforms allow remote regulators, quality auditors, or corporate HQ to access real-time batch data, deviations, and compliance records anywhere in the world. This creates a transparent audit trail that can be inspected years after production.
5.3 Why the Industry Is Paying Attention
Pharma’s growing interest in micro-factories is driven by three converging forces:
- Rise of Personalized Medicine: Cell and gene therapies, mRNA vaccines, and individualized oncology treatments don’t fit well into the mass-production model. They require small, rapid, tailored runs.
- Supply Chain Resilience: COVID-19 and geopolitical disruptions revealed the fragility of global supply chains. Microfactories cut out reliance on long, vulnerable distribution routes.
- Speed to Patient: A micro-factory can turn a digital prescription into a finished batch within hours. Imagine a cancer patient receiving a custom vaccine the same day their tumor biopsy is sequenced.
For regulators, this decentralized model may feel radical, but for patients, it could mean faster access, lower risk, and more equitable distribution of advanced therapies.
5.4 Real-World Pilots Already in Motion
MIT’s Langer Lab has demonstrated personalized cancer vaccines produced in microfactories within 24 hours, proving feasibility in precision oncology.
BioNTech’s “BioNTainers” modular mRNA vaccine production facilities are being deployed to Africa, ensuring low- and middle-income countries can manufacture COVID-19 and future vaccines locally.
DARPA’s Battlefield Medicine Program is investing in portable pharmaceutical synthesis systems that can make antibiotics, antidotes, or emergency treatments directly in war zones.
Each case demonstrates a core truth: micro-factories are not experimental curiosities; they are becoming strategic assets.
5.5 Challenges Before Mainstream Adoption
No disruptive technology arrives without hurdles. Key challenges include:
- Regulatory Harmonization – GMP frameworks were built for permanent facilities. Inspecting, certifying, and licensing modular units requires new paradigms.
- Validation Complexity – Standardized modules may face unique environmental or supply-chain constraints at each site, demanding tailored qualification.
- Skilled Workforce Availability – Even with high automation, microfactories require local staff trained in advanced bioprocesses and digital systems.
- Cybersecurity Risks – With IoT integration comes vulnerability. A cyberattack could halt production or even corrupt quality data, a risk regulators will scrutinize heavily.
5.6 Looking Ahead: Where Microfactories Meet VBE
The most transformative vision lies in integration with Virtual Bioequivalence (VBE). Picture this:
- Step 1: A company uses VBE simulations to prove that a generic cancer drug meets bioequivalence criteria without a human trial.
- Step 2: The validated model parameters are uploaded directly into a microfactory’s control system.
- Step 3: Within 48 hours, the microfactory manufactures the formulation, with AI-PAT certifying compliance in real time.
- Step 4: A digital certificate of analysis is instantly transmitted to regulators, bypassing weeks of manual QC.
This convergence of simulation + digital manufacturing could redefine not just BE trials, but the very philosophy of how medicines are developed, approved, and delivered.
And crucially, it’s not science fiction. With regulatory frameworks maturing and micro-factory pilots scaling, a five-to-seven-year horizon for widespread adoption is realistic.
Conclusion: A Paradigm Shift in Drug Development
Virtual Bioequivalence isn’t just replacing trials, it’s rewriting the rules of drug development, where science moves at the speed of thought, and healing reaches patients before delays ever begin.
In every era, there comes a moment when science leaps so profoundly that the old ways seem almost unimaginable. Today, we stand at that precipice. The shift from traditional human-driven bioequivalence trials to virtual simulations powered by data, intelligence, and precision is not just an evolution; it is a revolution quietly reshaping pharmaceutical science.
For decades, we relied on crowded clinical wards, sleepless volunteers, and endless vials of blood to answer a single question: Does this drug work the same? But now, that question can often be answered without a single needle, without a single ward, and sometimes without a single patient. With Virtual Bioequivalence (vBE) and Model-Informed Bioequivalence (MIBE), what once took years can now unfold in days.
The science speaks for itself. Whether it’s acyclovir’s food-effect modeling, tofacitinib’s pediatric dosing predictions, colonic release formulations, inhalation generics, or mRNA stability simulations, the results are stunningly consistent: virtual models don’t just replicate human trials, they predict them, sometimes with even greater precision. Each case study tells the same story: a new frontier where intelligent modeling meets clinical reality, and where computational insight isn’t replacing human expertise, but magnifying it.
Regulators are listening. The FDA, EMA, and WHO are no longer asking, “Is this acceptable?” Instead, the question has shifted to: “How strong is your model?” Initiatives like the MIDD Pilot Program and the ICH M13A guideline signal a future where validated models will become not the exception but the expectation. Tomorrow’s approvals will be judged not just by in vivo evidence, but by the strength of what’s proven in silico.
And then there are the AI-driven micro-factories, portable, modular, GMP-compliant powerhouses capable of producing complex drugs on-demand, right where patients need them most. Imagine this: a validated VBE model uploaded directly into a micro-factory’s control system, where the drug is synthesized, quality-tested in real time through AI-driven PAT, and released to patients within hours, complete with digital certification sent straight to regulators. This is no longer a dream; it’s a future just five to seven years away.
This isn’t about replacing humans with machines or science with simulations. It’s about liberating innovation from its old constraints. It’s about accelerating discovery without compromising safety, reaching patients without the delays of outdated logistics, and empowering scientists with tools that allow them to fail faster, innovate smarter, and heal sooner.
The age of long, exhausting trials, of mountains of data locked behind months of waiting, is coming to an end. In its place rises an era of intelligent medicine, faster, safer, and infinitely more precise. The transformation is here. And one day soon, we’ll look back at the days of endless wards, sleepless nights, and thousands of vials, and wonder how we ever did it any other way.
Because the future of medicine is no longer written only in blood samples and human trials.
It’s being simulated, optimized, and delivered, one algorithm, one micro-factory, and one patient at a time.
References
- Alotaiq, N., & Dermawan, H. (2024). Meta-Analysis of PBPK-Based Virtual Bioequivalence Studies: Implications for Regulatory Acceptance. Pharmaceutics, 16(4), 510–528.
- Turner, D.B., et al. (2023). Application of PBPK Modelling for Pediatric Dose Adjustments in Tofacitinib. Clinical Pharmacokinetics, 62(9), 1123–1138.
- Chaturvedi, R., et al. (2022). Simulation of Food Effects on Acyclovir Absorption Using PBPK Models. AAPS Journal, 24(6), 122–135.
- · U.S. Food and Drug Administration. (2023). Model-Informed Drug Development Pilot Program: Interim Report.
- · European Medicines Agency. (2022). Reflection Paper on the Use of PBPK Modelling for Bioequivalence Assessment.
- · ICH M13A Draft Guideline. (2024). Bioequivalence for Immediate-Release Solid Oral Dosage Forms.
- · World Health Organization. (2023). WHO Model Biowaiver Guidelines for Solid Oral Dosage Forms.
- · R. Langer Lab, Massachusetts Institute of Technology – Modular Vaccine Manufacturing Publications.
- · BioNTech. (2023). BioNTainer: Decentralized mRNA Vaccine Production Units.
- · U.S. Department of Defense, DARPA – Battlefield Medicine Initiative.
- · FDA. (2022). Advances in Continuous Manufacturing and PAT Implementation.
- · International Society for Pharmaceutical Engineering (ISPE). (2023). Guidelines for Decentralized Manufacturing Models.