Pharmacovigilance, the science of detecting, assessing, understanding, and preventing adverse drug effects, is crucial for patient safety and public health. This article explores its evolution, key concepts, methodologies, and regulatory frameworks. It addresses current challenges, such as underreporting and data complexity, while highlighting future trends driven by AI, big data, and real-world evidence. By continuously adapting to new knowledge and technology, pharmacovigilance remains at the forefront of safeguarding the safe and effective use of medicinal products globally.
Introduction
New medicines and vaccines offer significant health benefits but carry inherent risks, including adverse drug reactions (ADRs). Pharmacovigilance (PV) and drug safety monitoring are essential for ensuring the safe use of these products throughout their lifecycle. PV is a dynamic discipline focused on continuously monitoring medicinal products to identify, assess, understand, and prevent adverse effects, thereby promoting rational and safe medicine use. Drug safety monitoring, a closely related field, typically focuses on the immediate collection and reporting of adverse events.
Defining Pharmacovigilance and Drug Safety
While often used interchangeably, pharmacovigilance (PV) and drug safety have distinct nuances. The World Health Organization (WHO) defines PV as the science and activities relating to the detection, assessment, understanding, and prevention of adverse effects or any other medicine/vaccine-related problem. This highlights PV as a proactive, scientific discipline covering the entire drug lifecycle, aiming to identify, understand, and prevent safety issues. It continuously evaluates a product’s benefit-risk profile.
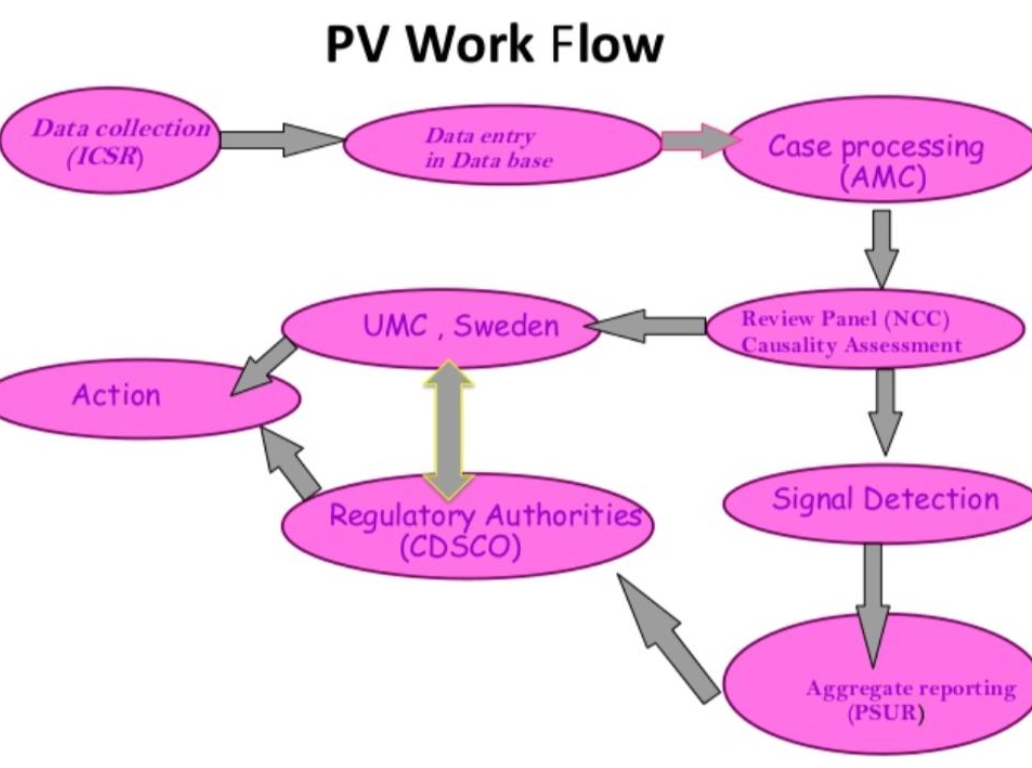
Drug Safety, conversely, focuses on the immediate, operational aspects of managing adverse events. It primarily involves the collection, processing, and reporting of individual adverse drug reactions (ADRs) from clinical trials or post-marketing surveillance. It is a reactive component of PV, providing raw data for broader safety assessments.
Comparison of Pharmacovigilance and Drug Safety
| Feature | Pharmacovigilance | Drug Safety |
| Focus | Proactive: Signal interpretation, risk management | Reactive: Compliance reporting, adverse effects |
| Scope | Broader: Entire drug lifecycle, risk- benefit | Narrower: Adverse drug effects during treatment |
| Activities | Detection, assessment, understanding, prevention | Collection, processing, reporting of ADRs |
| Goal | Continuous safety monitoring, public health | Ensuring immediate safety, regulatory compliance |
| Timeframe | Long-term, continuous | Broader: Entire drug lifecycle, risk-benefit |
In essence, drug safety is a subset of the broader pharmacovigilance discipline. PV utilizes drug safety data for deeper analysis, trend identification, and informed decision-making on overall drug safety. The field has evolved from a purely reactive drug safety approach to a comprehensive, proactive pharmacovigilance paradigm, driven by increasing pharmaceutical complexity and the need for robust patient protection.
Historical Milestones: The Impact of the Thalidomide Tragedy
The Thalidomide tragedy in the mid-20th century profoundly reshaped pharmacovigilance. Marketed in the late 1950s as a sedative, thalidomide was widely prescribed, including to pregnant women. By the early 1960s, a disturbing link emerged between thalidomide use and severe birth defects, particularly phocomelia (malformed limbs). Dr. Widukind Lenz and Dr. William McBride identified this connection.
This global catastrophe exposed critical flaws in drug development and regulation, which had focused on acute toxicity and efficacy, neglecting long-term or teratogenic effects. Post-marketing surveillance was informal.
The tragedy spurred significant reforms:
- Stricter Drug Regulations: Laws requiring comprehensive pre-clinical and clinical testing, including teratogenicity studies, were enacted. The U.S. Kefauver-Harris Amendments (1962) mandated proof of efficacy and safety and adverse event reporting.
- Formal Pharmacovigilance Systems: Continuous post-market safety monitoring became essential. National pharmacovigilance centers and spontaneous reporting systems were established. The WHO Programme for International Drug Monitoring (WHO PIDM) began in 1968 to facilitate international safety information exchange.
- Emphasis on Post-Marketing Surveillance: The event highlighted that unforeseen risks could emerge after widespread use, making ongoing surveillance crucial.
The Thalidomide tragedy transformed drug safety from a reactive to a proactive pharmacovigilance model. Its lessons continue to shape current pharmacovigilance practices, underscoring the paramount importance of patient safety throughout drug development and use.
The Importance and Scope of Pharmacovigilance
Pharmacovigilance is fundamental to modern healthcare, ensuring the ongoing safety and efficacy of medicinal products. Its importance extends beyond regulatory compliance, influencing drug development, clinical practice, and patient care. The scope is vast, encompassing continuous monitoring, evaluation, and risk management of drug use.

The Importance of Pharmacovigilance
Pharmacovigilance addresses the limitations of pre-market clinical trials, which are conducted under controlled conditions with limited populations and durations. These trials may miss rare, long-term, or subgroup-specific adverse drug reactions (ADRs). PV systematically captures and analyzes real-world data to identify and manage previously unknown risks.
Key aspects of PV’s importance include:
- Protecting Patients: Its primary goal is to minimize drug-related harm by identifying and managing risks, enabling informed treatment decisions.
- Improving Public Health: By ensuring safe medicine use, PV reduces drug-related morbidity and mortality, enhancing public health and trust in healthcare.
- Enhancing Benefit-Risk Profile: PV continuously re-evaluates a drug’s benefit-risk balance, ensuring benefits outweigh risks and allowing for adjustments as new safety information emerges.
- Supporting Regulatory Decisions: PV data provides regulators with evidence for informed decisions on drug labeling, risk management, and market actions.
- Promoting Rational Use: By disseminating comprehensive safety information, PV encourages rational and safe medicine use by healthcare professionals and patients.
The Scope of Pharmacovigilance
The scope of PV covers the entire product lifecycle, involving:
- Data Collection and Management: Systematic collection of safety data from spontaneous reports, clinical trials, epidemiological studies, and literature. Data is managed in specialized databases (e.g., FAERS, VigiBase).
- Signal Detection and Assessment: Actively searching for new or changing information suggesting a causal link between an adverse event and a drug. Signals are rigorously assessed for clinical significance.
- Risk Management and Minimization: Developing and implementing risk management plans (RMPs) or risk evaluation and mitigation strategies (REMS) to minimize identified risks. Measures include updated labeling, educational materials, and restricted distribution.
- Communication and Information Dissemination: Timely and transparent dissemination of safety information to healthcare professionals, patients, the public, and regulators via alerts and advisories.
- Regulatory Compliance: Adherence to national and international regulations and guidelines to maintain system integrity.
- In summary, pharmacovigilance is crucial for the safe and effective use of medicines. Through continuous monitoring and evaluation, it protects patients, improves public health, and fosters a culture of safety in the pharmaceutical sector.
Methods and Approaches in Drug Safety Monitoring
Effective drug safety monitoring employs diverse methods to understand a product’s safety profile, ranging from passive data collection to active surveillance and targeted studies.
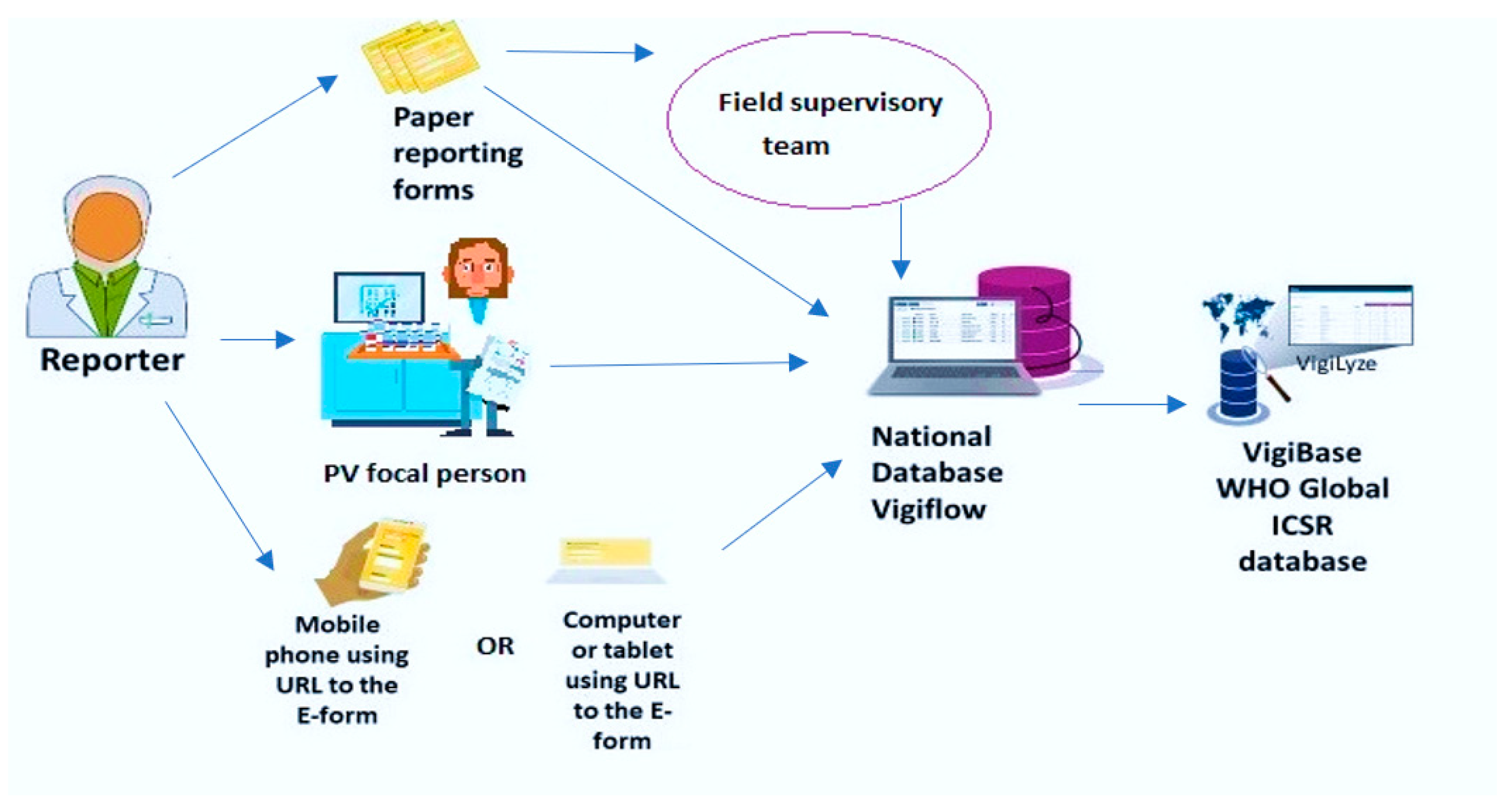
Spontaneous Reporting Systems
Spontaneous reporting, or passive surveillance, is the foundation of pharmacovigilance. It involves voluntary submission of suspected adverse drug reactions (ADRs) by healthcare professionals and patients to national centers or companies. These Individual Case Safety Reports (ICSRs) are vital for early signal generation, especially for rare ADRs. Examples include FDA FAERS, EudraVigilance, and WHO’s VigiBase. Limitations include underreporting and reporting bias, but they remain crucial for continuous monitoring.

Active Surveillance
Active surveillance systematically collects ADR data from defined populations, overcoming underreporting. Methods include:
- Cohort Event Monitoring (CEM): Following a cohort of patients on a specific drug to collect all adverse events.
- Prescription-Event Monitoring (PEM): A UK-based variant of CEM, collecting ADR data from general practitioners for newly marketed drugs.
- Patient Registries: Organized systems collecting standardized data on patients with specific conditions or drug exposures, providing long-term safety data.
Targeted Studies
When a safety signal emerges, targeted epidemiological studies investigate the association and establish causality:
- Case-Control Studies: Retrospective studies comparing drug exposure in individuals with and without an ADR.
- Cohort Studies: Prospective studies following groups with different drug exposures to observe ADR incidence.
- Randomized Controlled Trials (RCTs): Primarily for efficacy, larger RCTs also provide safety data, and post-marketing RCTs can investigate specific safety concerns.
Data Mining and Signal Detection
Advanced computational techniques, including data mining algorithms, analyze large ICSR databases to identify disproportionate reporting of drug-ADR pairs, indicating potential safety signals. Statistical methods like Proportional Reporting Ratio (PRR) and Reporting Odds Ratio (ROR) are commonly used.
Electronic Health Records (EHRs) and Real-World Data (RWD)
EHRs and other RWD sources (e.g., claims databases, social media) offer insights into drug use and safety in routine clinical practice, complementing traditional trials. RWD aids both signal detection and confirmatory studies, providing a comprehensive safety profile in diverse populations.
Risk Management Plans (RMPs) and Risk Evaluation and Mitigation Strategies (REMS)
For drugs with significant risks, regulatory authorities require RMPs or REMS. These plans outline activities to identify, characterize, prevent, or minimize risks, including updated labeling, educational materials, and restricted distribution. These diverse methods, when integrated within a robust pharmacovigilance system, enable continuous monitoring and timely interventions. The overall process is a continuous iterative cycle that ensures ongoing commitment to drug safety throughout a product’s lifecycle.
Regulatory Frameworks and International Guidelines
The global pharmaceutical landscape is governed by evolving regulatory frameworks and international guidelines, ensuring medicinal product safety, efficacy, and quality. Pharmacovigilance is integral to these structures, with various authorities setting standards and fostering harmonization.
International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH)
The ICH is a collaborative initiative of regulatory authorities and the pharmaceutical industry from Europe, Japan, and the United States. It aims to harmonize technical guidelines for pharmaceutical registration, reducing redundant testing and streamlining drug development. Key ICH pharmacovigilance guidelines include:
- ICH E2A & E2D: Standardize expedited reporting of individual case safety reports (ICSRs) from clinical trials and post-marketing, respectively.
- ICH E2B: Specifies standardized data elements and electronic format for ICSR transmission.
- ICH E2C: Outlines content and format for Periodic Benefit-Risk Evaluation Reports (PBRERs).
- ICH E2E: Provides recommendations for pharmacovigilance planning.
World Health Organization (WHO)
The WHO leads global public health, including pharmacovigilance. Through its Programme for International Drug Monitoring (WHO PIDM), it maintains VigiBase, the world’s largest ICSR database. The WHO also develops norms, standards, and guidelines, providing technical assistance to countries, building global drug safety capacity.
European Medicines Agency (EMA)
The EMA is the EU agency responsible for scientific evaluation, supervision, and safety monitoring of medicines. The EU has a comprehensive pharmacovigilance framework, strengthened by 2012 legislation. The EMA enforces Good Pharmacovigilance Practices (GVP), detailed guidelines covering all aspects of PV, mandatory for marketing authorization holders in the EU.

U.S. Food and Drug Administration (FDA)
The FDA is the primary U.S. drug safety regulatory body. It sets extensive regulations and guidance for pharmacovigilance, including mandatory adverse event reporting (e.g., via MedWatch, feeding into FAERS), and requires Risk Evaluation and Mitigation Strategies (REMS) for high-risk drugs. The FDA continuously monitors safety signals and takes regulatory actions like label changes or product withdrawals.
These dynamic regulatory frameworks and international guidelines collectively aim to establish a harmonized, robust, and transparent global pharmacovigilance system, ensuring safe and effective medicine use worldwide.
Challenges and Future Trends in Pharmacovigilance
Pharmacovigilance faces ongoing challenges due to the dynamic nature of drug development and increasing data complexity. However, these challenges also drive innovation, leading to transformative future trends in drug safety monitoring.
Current Challenges
- Underreporting of ADRs: Persistent underreporting leads to incomplete safety profiles and delayed signal detection.
- Big Data: The explosion of data from diverse sources (e.g., spontaneous reports, EHRs, social media) creates challenges in managing, processing, and extracting insights from vast, heterogeneous datasets.
- Data Quality: Inconsistent terminology, errors, and lack of standardization compromise analysis reliability.
- Signal Prioritization: Identifying true safety signals amidst noise and false positives requires sophisticated tools and expert judgment.
- Globalization: Differences in reporting standards and regulations across countries create operational complexities.
- Emerging Therapies: Novel therapies like gene and cell therapies pose unique PV challenges due to their complex mechanisms and potential for delayed effects.
- Resource Constraints: Many PV systems, especially in developing countries, face limitations in skilled personnel, technology, and funding.
Future Trends
PV systems are evolving rapidly, driven by technology and a focus on proactive, data-driven approaches.
- Artificial Intelligence (AI) and Machine Learning (ML): AI/ML will revolutionize PV by automating tasks (e.g., case intake, data extraction via NLP) and analyzing vast datasets to identify complex patterns, predict adverse events, and detect drug-drug interactions, leading to faster and more accurate signal detection.
- Big Data Analytics: Effective big data analytics will integrate and analyze real-world data (RWD) from diverse sources (EHRs, claims, registries, social media) to provide a comprehensive understanding of drug safety in real-world settings, enabling proactive risk identification and nuanced benefit-risk assessment.
- Real-World Evidence (RWE): RWE, derived from RWD, is gaining regulatory acceptance. Future PV will increasingly leverage RWE to confirm safety signals, evaluate risk management, and support regulatory decisions, offering a complete picture of drug performance.
- Automation: Automation of repetitive tasks (data entry, report generation) will free PV professionals for higher-value activities like signal analysis and risk assessment.
- Patient-Centric PV: Greater emphasis will be placed on patient input, including direct patient reporting and use of patient-generated health data (mHealth apps, wearables).
- Advanced Signal Detection: Continuous development of statistical and computational methods will improve detection of rare, delayed, or complex adverse events.
- Blockchain: While nascent, blockchain offers potential for enhancing data integrity, security, and traceability in PV.
These trends point to a future PV system that is more proactive, efficient, data-driven, and patient-centric, ultimately enhancing patient safety and informing healthcare decisions globally.
Conclusion
Pharmacovigilance and drug safety monitoring are essential for ensuring that the benefits of medicinal products outweigh their risks. The discipline has evolved significantly, driven by events like the Thalidomide tragedy, which highlighted the need for continuous drug safety surveillance. PV encompasses systematic data collection, adverse drug reaction assessment, proactive signal detection, and robust risk management. These activities are guided by national and international regulatory frameworks from bodies like ICH, WHO, EMA, and FDA, all striving for global harmonization and patient protection.
Despite challenges such as underreporting, data complexity, and adapting to novel therapies, PV is undergoing transformative innovations. The future of PV is shaped by AI and machine learning for enhanced signal detection, big data analytics for real-world insights, and increased reliance on real-world evidence. A growing emphasis on patient-centric approaches and emerging technologies like blockchain and genomics promises more precise, efficient, and responsive individualized safety monitoring.
In an era of rapid medical advancements, PV remains critical. By embracing technology, fostering international collaboration, and committing to patient safety, PV will continue to evolve, ensuring medicines improve health while minimizing harm. This continuous vigilance is a fundamental ethical imperative, safeguarding public health and fostering trust in the therapeutic landscape.
References
- European Medicines Agency. (2023). Pharmacovigilance Legislation.
- Harpaz, R., et al. (2016). Real-world evidence for assessing adverse drug reactions in pharmacoepidemiology. Clinical Pharmacology & Therapeutics.
- Pirmohamed, M., et al. (2014). Adverse drug reactions as a cause of hospital admission: prospective analysis of 18,820 patients. BMJ.
- U.S. Food and Drug Administration. (2023). MedWatch: The FDA Safety Information and Adverse Event Reporting Program.
- World Health Organization. (2002). The International Drug Monitoring: The Role of National Centres. WHO.
