Introduction
Pharmaceutical solubilizers are excipients that enhance the solubility and dissolution rate of poorly water-soluble drugs, thereby improving their bioavailability. This article discusses the types, mechanisms, and applications of pharmaceutical solubilizers in pharmaceutical formulations. Poor solubility is a major challenge in drug development, affecting nearly 40% of marketed drugs and up to 90% of new chemical entities.
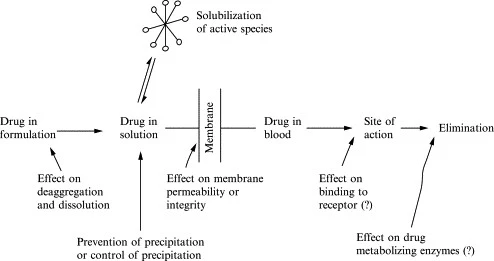
Functional mechanisms
The functional mechanism depends upon the nature of the solubilizers. Surfactants typically reduce surface tension and form micelles within the solvent system for the solute to dissolve or disperse. The mechanism of pharmaceutical solubilizers with surfactants is associated with a favorable interaction of the insoluble agent and the interior core of the pharmaceutical solubilizer assembly (e.g., micelles).
Hydroalcoholic solvents such as propylene glycol enable solubilization based on changes in the dielectric constant of the medium. Other types of pharmaceutical solubilizers use a range of polymeric chains that interact with hydrophobic molecules to increase solubility by dissolving the insoluble agent into the polymeric chains. Surface-active agents facilitate solubilization, micellization, or wetting of the solute by enhancing the spreading and penetrating properties of a liquid, thereby lowering its surface tension.
In other cases, unique hydrophobic sites that are capable of forming inclusion complexes are present. Hydrophobic complexes result from an energetically favored tendency for hydrophobic entities to interact with the solubilizing agent. Inclusion complexes such as cyclodextrins require sufficient opportunity for a solute molecule to favorably interact with the complexing agent cavity based on molecular size and associated hydrophobic-hydrophilic interactions. Cavity dimensions are an important consideration in the ability of a cyclodextrin or modified cyclodextrin to accommodate a drug substance molecule.
Types of Pharmaceutical Solubilizers
1. Surfactants
- Examples: Polysorbates (Tween®), sodium lauryl sulfate (SLS), Cremophor® EL
- Mechanism: Form micelles to encapsulate hydrophobic drugs.
- Applications: Used in oral, injectable, and topical formulations.
2. Cyclodextrins
- Examples: β-cyclodextrin, hydroxypropyl-β-cyclodextrin (HPβCD), sulfobutyl ether-β-cyclodextrin (SBEβCD)
- Mechanism: Form inclusion complexes with drug molecules.
- Applications: Common in oral and parenteral formulations.
3. Co-Solvents
- Examples: Ethanol, propylene glycol, polyethylene glycol (PEG)
- Mechanism: Alter solvent polarity to enhance drug solubility.
- Applications: Used in liquid dosage forms (syrups, injectables).
4. Lipid-Based Systems
- Examples: Medium-chain triglycerides (MCTs), phospholipids (lecithin)
- Mechanism: Enhance solubility via emulsification or micelle formation.
- Applications: Soft gelatin capsules, lipid nanoparticles.
5. Polymers
- Examples: Polyvinylpyrrolidone (PVP), hydroxypropyl methylcellulose (HPMC)
- Mechanism: Inhibit drug precipitation and stabilize supersaturation.
- Applications: Solid dispersions, amorphous formulations.

Mechanisms of Solubilization
- Micellar Encapsulation (Surfactants)
- Inclusion Complexation (Cyclodextrins)
- Polarity Modulation (Co-Solvents)
- Lipid Dispersion (Emulsions, Liposomes)
- Amorphous Stabilization (Polymer-Based Systems)
Challenges and Considerations
- Safety & Toxicity: Some solubilizers (e.g., Cremophor EL) may cause hypersensitivity.
- Drug-Excipient Interactions: May affect stability or release profile.
- Regulatory Compliance: Must meet pharmacopeial standards (USP, EP).
- Cost & Scalability: Cyclodextrins and lipid systems can be expensive.
Conclusion
Pharmaceutical solubilizers are considered one of the essential excipients for overcoming solubility challenges and enhancing drug bioavailability. The selection of an appropriate solubilizer depends on the drug’s physicochemical properties, formulation requirements, intended application, and route of administration.
Bringing it all together
As we wrap up this series on pharmaceutical excipients, it’s clear that each article has taken a deeper look into their properties, types, and uses, offering insights, practical tips, and thought-provoking ideas.
Thank you for following along. If this series sparked new ideas or left you with questions, we’d love to hear from you. And if you missed any part, be sure to revisit the earlier articles for a complete picture.
Stay curious—and stay tuned for more!
References
- Handbook of Pharmaceutical Excipients – Rowe, Sheskey, & Quinn.
- Drug-Like Properties: Concepts, Structure Design, and Methods – Li & Di.
- Pharmaceutical Solubility Enhancement Using Cyclodextrins – Loftsson & Brewster.
- USP/NF Guidelines on Solubilizing Agents.
- USP/NF Excipient Performance <1059>

