In the world of pharmaceuticals, developing an effective drug doesn’t stop at selecting the drug molecule. One of the key challenges is ensuring that the drug is released from its dosage form (such as oral solid dosage forms) and becomes available in the body to exert its intended therapeutic effect. This is where drug dissolution testing and drug solubility play a vital role.

Dissolution as a term
refers to the process by which a solid substance (like a drug) dissolves in a solvent (like the fluid in your gastrointestinal tract). In pharmaceutical sciences, it is used to study how quickly and efficiently a drug is released from its formulation and made available for absorption, the rate and extent of release
This article aims to introduce the fundamental concepts of dissolution testing and drug release. We’ll explore how the test works, why it’s important, the apparatus and methods used, and Types of drug release.
Whether you are a student, a junior analyst, or a formulator just starting your journey in pharmaceutical sciences, understanding dissolution is a cornerstone of developing safe and effective medicines.
The process of dissolution, what is happening
When an immediate-release tablet or other solid dosage form is introduced into a beaker of water or the gastrointestinal tract, the following steps successively take place.
- Disintegration: breaking of a tablet into granules.
- Disaggregation: breaking of these granules into individual particles.
- Dissolution: Finally, particles dissolve, releasing the active ingredient into the solution.
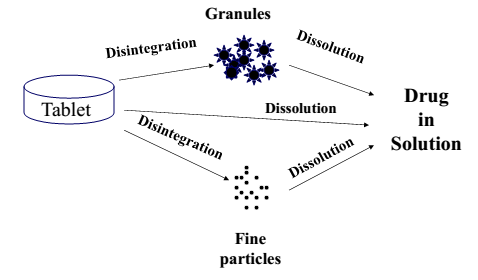
Drug Dissolution vs Solubility vs Drug Release:
| Term | Definition |
| Solubility | It is more of a property; it is the maximum amount of drug that can dissolve in a specific solvent. |
| Dissolution | The rate at which the drug actually dissolves over time. |
| Drug release | The overall process by which the drug becomes available for absorption, which may include dissolution but also depends on dosage form design. |
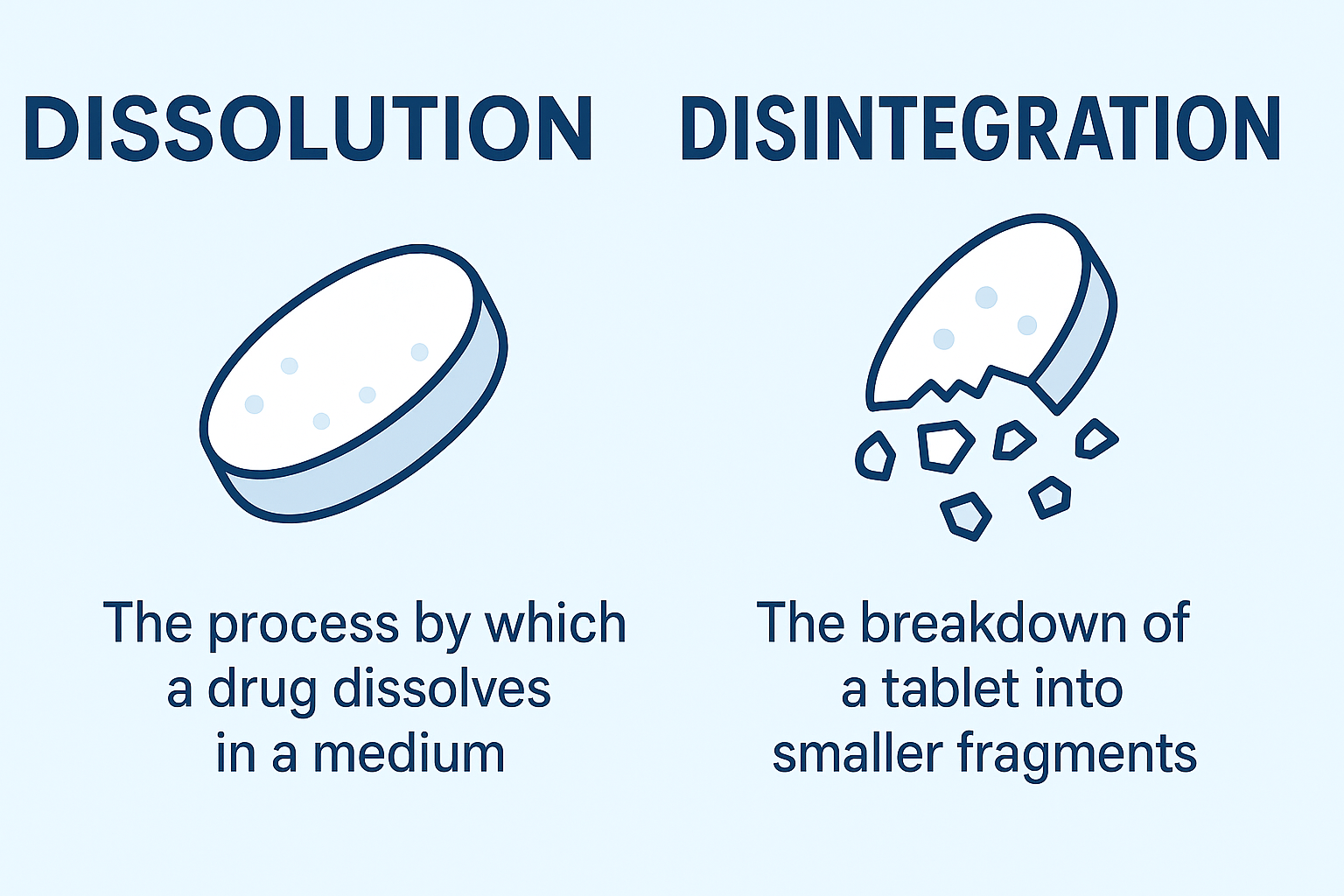
A quick important note: A drug may have good solubility but poor dissolution due to large particle size or compact tablet structure.
In-Vitro vs In-Vivo
- In-vitro Dissolution testing: is performed in the laboratory using standardized and calibrated apparatuses and controlled conditions.
- In-vivo Dissolution: occurs inside the human body and can be affected (positively or negatively) by various physiological factors, such as stomach pH, food intake, and gastrointestinal motility.
Types of Drug Release Profiles
1) Immediate Release (IR)
In this type, the tablet disintegrates and the active ingredient is released immediately after administration, usually within minutes. The goal is to achieve a fast onset of action, which is intended for drugs intended for quick relief, such as painkillers or antipyretics (fever reducers).
- Example: Paracetamol tablets
- Key Feature: No delay; rapid disintegration and dissolution
- Dissolution Goal: NLT 80% drug release within 30 minutes (varies according to the pharmacopeia)
2) Extended Release (ER):
Also include sustained-release or controlled-release dosage forms; these dosage forms are designed and formulated to release the drug gradually over an extended period. This maintains therapeutic drug levels longer and lessens the frequency of dosing.
- Example: Metformin ER for diabetes
- Key Feature: Steady, prolonged drug release
- Advantages: Improved patient convenience and Reduced side effects from peak drug concentrations.
3) Delayed Release (DR):
Delayed release dosage forms release the drug either after a specific time or in a specific environment, such as the intestine instead of the stomach. These are often used to protect the drug against stomach acid or to avoid gastric irritation.
- Example: Enteric coated aspirin tablets.
- Key Feature: Little or no release in the stomach, drug release starts in the intestine.
- Mechanism: pH sensitive coatings dissolve only at higher pH values (as in the small intestine).
4) Pulsatile Release:
This is a less common and specialized system where the drug is released in bursts, separated by time delays. It’s often designed to mimic the body’s natural rhythms or to target drug release at a specific time (for example for treating nighttime asthma or early-morning arthritis pain).
- Example: Some circadian-based drug delivery systems.
- Key Feature: One or more drug “pulses” at defined time intervals.
Visualizing Release Profiles
A simple way to understand these types is through release curves:
- Immediate release: steep slope, rapid rise.
- Extended release: gradual, consistent slope.
- Delayed release: flat line (lag phase), then steep rise.
- Pulsatile: step-like bursts.
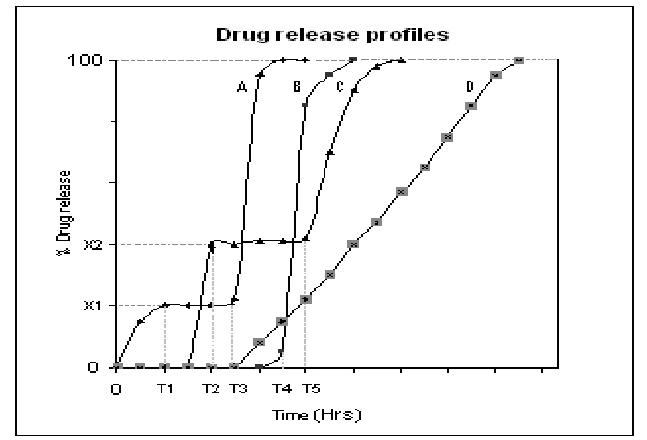
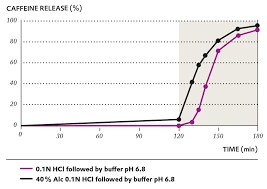
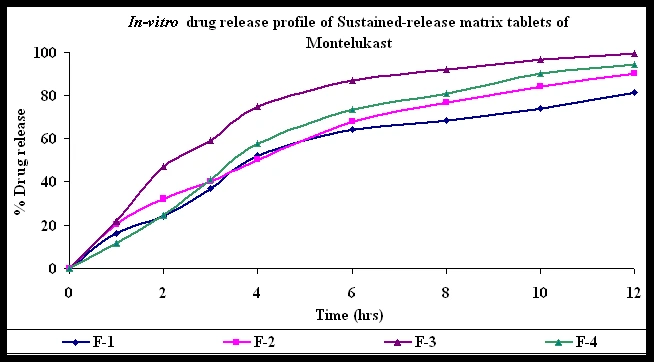
Apparatuses and Methods
To investigate how a drug dissolves and releases the API in controlled conditions, scientists use standardized and calibrated dissolution test equipment and procedures. These methods are important for comparing formulations, ensuring quality, and predicting how a drug will behave inside the human body.
The equipment assembly consists of the following: a vessel, which may be covered, made of glass or other inert, transparent material, a motor; a metallic drive shaft; and a cylindrical basket. The vessel is partially immersed in a suitable water bath of any convenient size or heated by a suitable device such as a heating jacket. The water bath or heating device permits holding the temperature inside the vessel at 37 ±0.5° during the test and keeping the bath fluid in constant, smooth motion. Shaft and basket components of the stirring element are fabricated of stainless steel.
Overview of Dissolution Apparatus According to USP
The United States Pharmacopeia (USP) describes official dissolution apparatuses. The most commonly used are Apparatus I (Basket) and Apparatus II (Paddle). Let’s start discussing them:
Apparatus I (Basket)
- Structure: A metal mesh basket attached to a rotating shaft.
- Use: The dosage form (usually a tablet or capsule) is placed inside the basket, then down into a vessel containing the dissolution medium.
- Rotation Speed: Commonly 100 rpm (differs based on formulation specs).
- Application: Suitable for capsules and tablets that may float.
Apparatus II (Paddle)
- Structure: A flat paddle rotates above the dosage form, which settles at the bottom of the vessel.
- Use: The paddle stirs the medium solution, allowing the dosage form to dissolve and release the drug gradually.
- Rotating Speed: commonly 50–75 rpm.
- Application: Commonly used for tablets that sink.
Other Apparatuses
For more specialized formulations, other USP apparatuses may be used:
- Apparatus III (Reciprocating Cylinder): For modified-release products.
- Apparatus IV (Flow-Through Cell): For poorly soluble or controlled-release drugs.
- Apparatus V–VII: 5 (paddle over disk), 6 (rotating cylinder), and 7 (reciprocating disk, less commonly used, for specific advanced dosage forms).
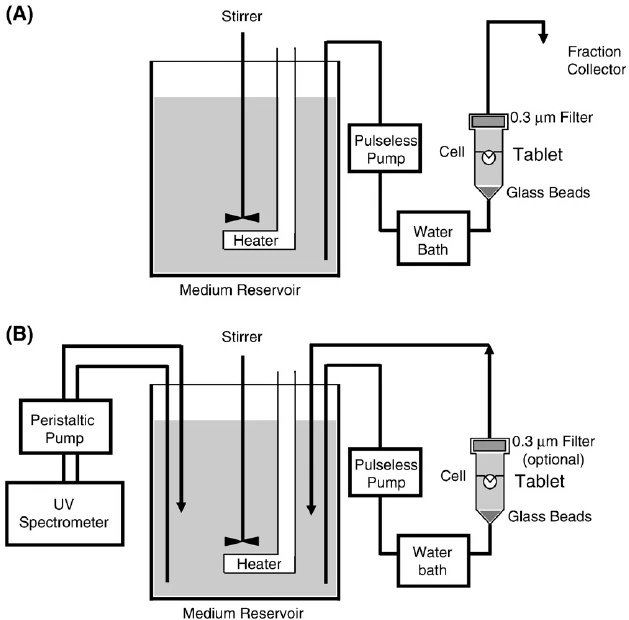
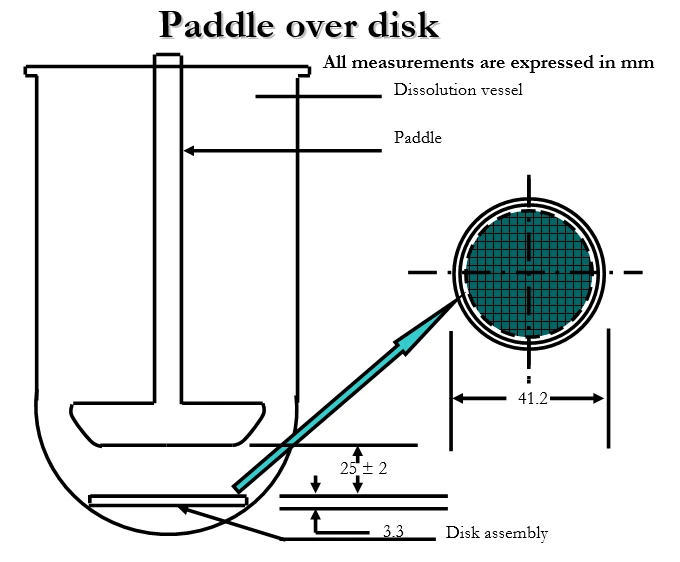
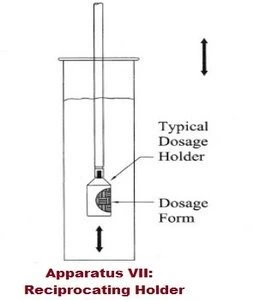
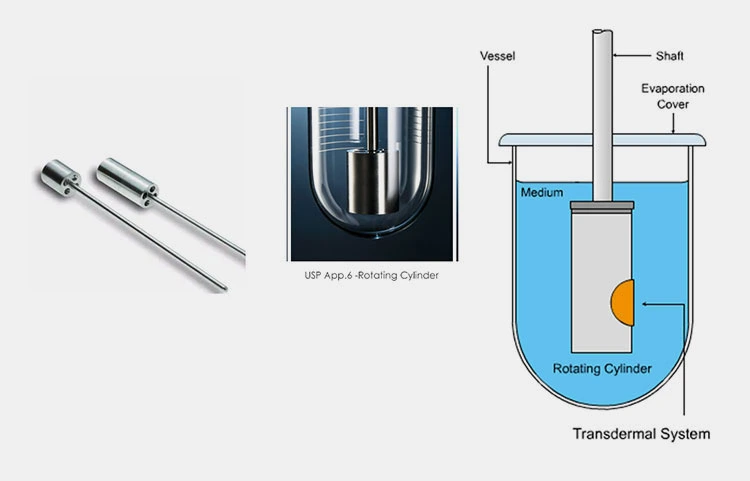
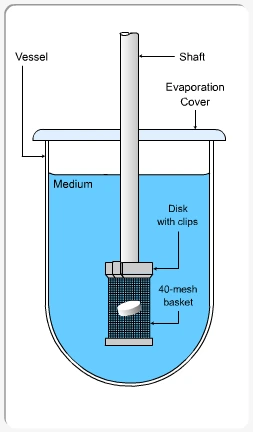
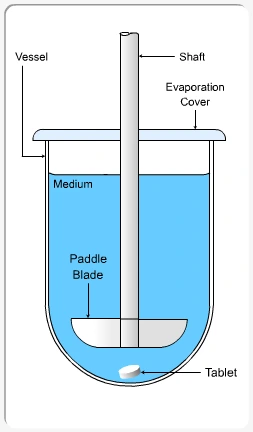
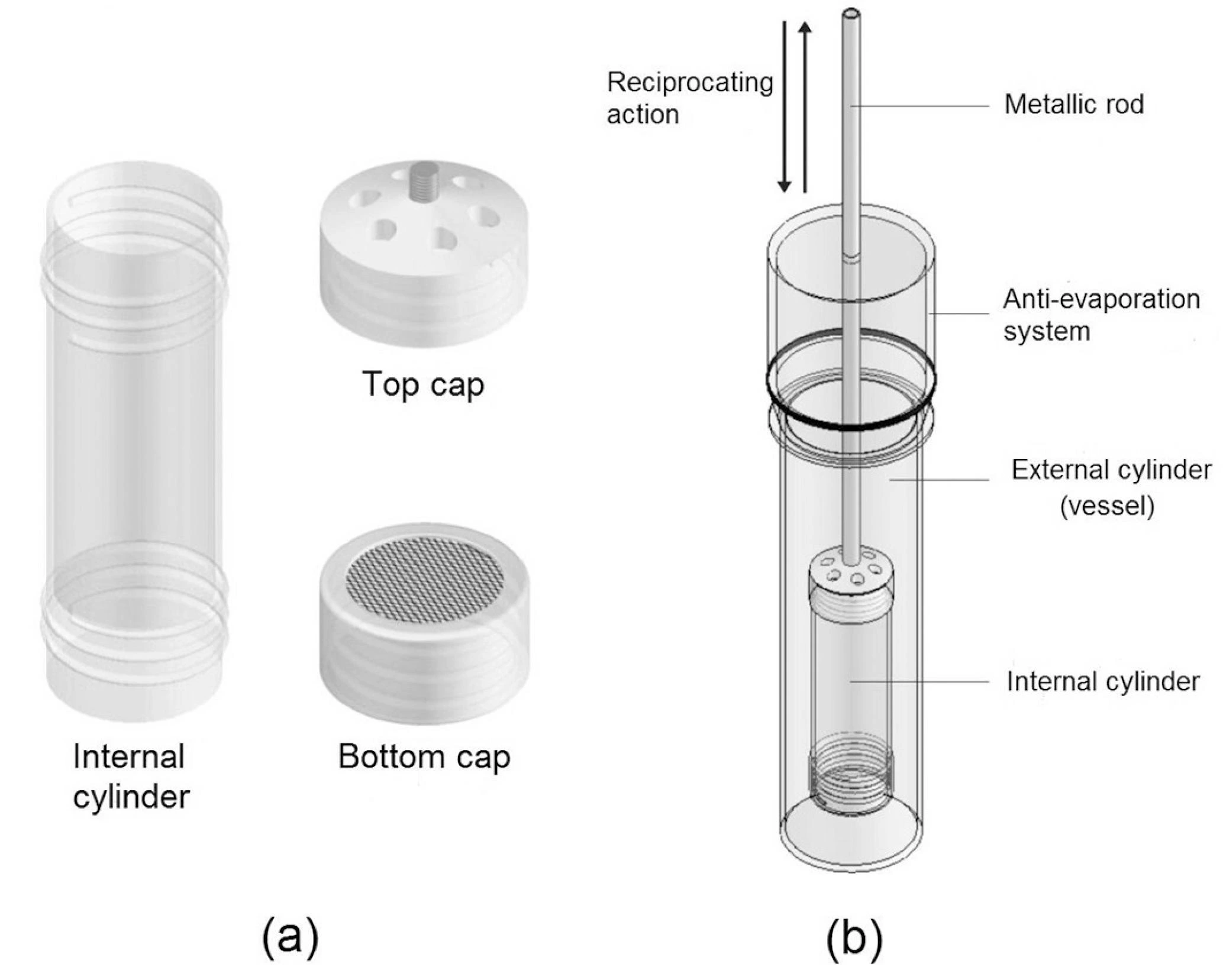
Summary of Dissolution test procedure:
- Prepare the medium and ensure correct pH and temperature, Place the stated volume of the Dissolution Medium (±1%) in the vessel of the specified apparatus according to the monograph.
- Place the dosage form in the basket or under the paddle.
- Start rotation at the specified speed.
- Withdraw samples at specific time intervals (e.g., 10, 15, 20, 30 minutes, .. as you procedure states).
- Filter and analyze samples using UV spectroscopy or HPLC using detectors such as RI or UV.
- Plot a dissolution profile (drug % released vs. time).
Official Guidelines for Dissolution Testing
Reading this article could be a great first step to start reading the official Guidelines for Dissolution testing:
- USP General chapter 711
- FDA guidance
Now let’s memorize what we’ve gone through in this article
We began by exploring what drug dissolution means and how it differs from related terms like solubility , and drug release. Through clear examples and analogies, we saw that a drug must dissolve before it can be absorbed—and that this simple principle drives much of the scientific work behind dosage form design.
We also examined the standard tools and methods used to perform dissolution testing, particularly the USP Apparatus I (Basket) and II (Paddle), which remain the workhorses of quality control labs around the world. Additionally, understanding the types of drug release profiles—immediate, extended, delayed, and pulsatile—helps formulators tailor medicines to achieve desired therapeutic outcomes and improve patient adherence.
Conclusion
Dissolution is a key process that links the physical dosage form to the drug’s actual performance inside the body. Understanding it lays the foundation for mastering pharmaceutical development, quality control, and regulatory compliance.
References:
- Lu JX, Tupper C, Gutierrez AV, Murray J. Biochemistry, Dissolution and Solubility. 2022 Sep 12. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. PMID: 28613752.
- https://www.usp.org/sites/default/files/usp/document/harmonization/gen-method/stage_6_monograph_25_feb_2011.pdf
- https://www.fda.gov/regulatory-information/search-fda-guidance-documents/dissolution-testing-immediate-release-solid-oral-dosage-forms

