Introduction
Imagine being able to identify a single molecule in a sea of millions — pinpointing its structure, quantity, and role in a complex system. That’s the power of Liquid Chromatography-Mass Spectrometry (LC-MS), a cutting-edge analytical tool that revolutionizes the way we explore the molecular world. By merging the precise separation abilities of liquid chromatography with the ultra-sensitive detection of mass spectrometry; the technique allows for the high-resolution separation, identification, and quantification of complex mixtures at very low concentrations. LC-MS has become indispensable across fields like pharmaceutical research, environmental analysis, clinical diagnostics, and proteomics. This article delves into the fascinating world of LC-MS, exploring how this technique works, why it’s so powerful, and what the future holds for molecular science.
LC-MS Instrumentation
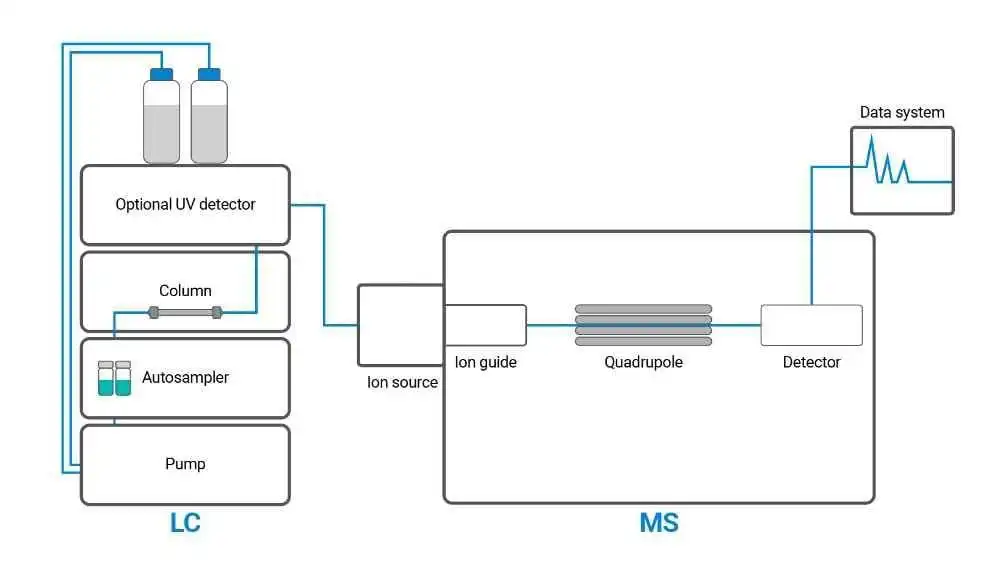
figure 1: LC-MS instrument diagram
An LC-MS system consists of several key components that work together to separate and identify compounds in a sample:
• Sample Injector: The sample is introduced into the system through an injector, often in the form of a small volume of liquid. In some systems, auto samplers are used for automated sample introduction, ensuring reproducibility and efficiency.
• Liquid Chromatograph: The liquid chromatograph is responsible for the separation of sample components. It typically includes a high-pressure pump, a chromatographic column, and a detector to monitor the elution profile.
• Ionization Source: The ionization source is a critical component in LC-MS, as it converts the separated compounds into charged ions.
• Mass Analyser: The mass analyser is responsible for sorting ions according to their m/z ratio.
• Detector: The detector measures the intensity of the ions produced and provides a signal corresponding to their abundance. Detectors such as the ion trap or the electron multiplier are commonly used.
Principles of Liquid Chromatography-Mass Spectrometry (LC-MS)
First, the sample is injected into a liquid chromatograph, where it’s carried by a liquid solvent through a column packed with a material that separates the mixture into individual components based on their chemical properties.
As each compound exits the column, it enters the mass spectrometer, where it is ionized—converted into charged particles.
These ions are then sorted and detected based on their mass-to-charge ratio, producing a spectrum that helps determine the identity and concentration of each compound.
Liquid Chromatography (LC)
Liquid chromatography is a separation technique used to isolate components of a mixture based on differences in their interactions with a stationary phase (usually a solid) and a mobile phase (typically a liquid). The sample is introduced into the chromatographic column, where it is subjected to partitioning between the stationary and mobile phases. The components of the sample will travel through the column at different rates, based on their chemical properties (e.g., polarity, size, charge), leading to their separation.
The most common type of liquid chromatography used in LC-MS is High-Performance Liquid Chromatography (HPLC). In HPLC, the sample is forced through a column under high pressure, enhancing the separation efficiency and reducing analysis time. The separation mechanism is largely based on the differential affinity of the sample components for the stationary phase, which can be manipulated through various chromatographic modes such as reversed-phase, ion-exchange, or size-exclusion chromatography.
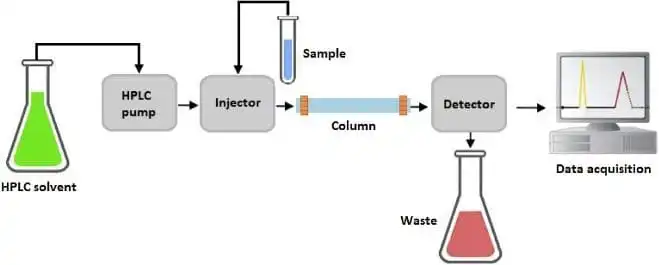
figure 2: LC diagram
Mass Spectrometry (MS)
Mass spectrometry is a technique used to measure the mass-to-charge ratio (m/z) of ions, providing detailed information about the molecular composition of a sample. The process involves three main stages:
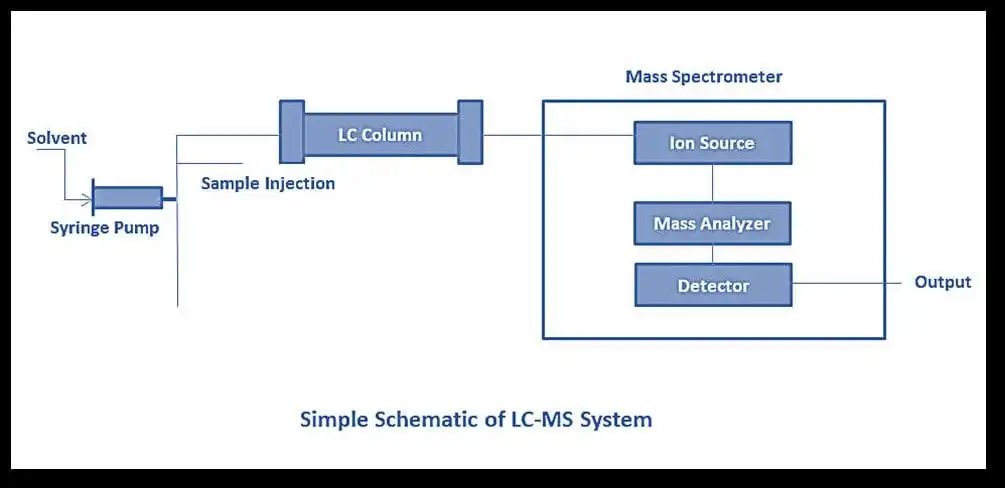
Figure 3: MS diagram
Ionization
In this stage, the sample molecules are converted into charged ions . In MS, ionization can be categorized into : hard ionization and soft ionization. The choice of ionization method depends on the nature of the sample as well as the purpose of the analysis.
| Comparison point | Hard ionization | Soft ionization |
| Energy | Apply high energy to the sample, causing extensive fragmentation. | Apply lower energy to ionize the sample, resulting in minimal fragmentation. |
| Fragmentation | The sample breaks into numerous fragments, providing structural information about the molecule with relatively low abundance of the molecular ion | Minimizes fragmentation, producing primarily molecular ions. |
| Application | Determining the molecular weight and structural information of smaller molecules. ( molecular weights below 600) | Suitable for analysing large, fragile molecules like proteins and peptides, where fragmentation is undesirable. |
| Examples | Electron Ionization ( EI) | Electrospray ionization (ESI), Matrix-assisted laser desorption/ionization (MALDI), and chemical ionization (CI). |
| Common method | EI is commonly used in Gas chromatography-MS for structural determination | ESI is commonly used in LC-MS for liquid samples while MALDI is used for solid samples |
The two most widely used ionization techniques are:
• Electrospray Ionization (ESI): ESI is used for the ionization of polar, thermally sensitive, and large biomolecules such as peptides, proteins, and nucleic acids. ESI produces multiply charged ions, which facilitates the detection of large molecules.Electrospray ionization (ESI) in mass spectrometry can be categorized into positive and negative ion modes, each offering different advantages for specific analysis
• Atmospheric Pressure Chemical Ionization (APCI): APCI is typically used for less polar compounds and is more suited for volatile and moderately polar molecules.
Mass analysis
- The generated ions from the ion source are separated and analysed in a mass analyser based on their m/z ratios. Only charged species can be manipulated by electric or magnetic fields in this step.
- Ion Acceleration in the mass analyser:
The ions are accelerated by an electric field to give them kinetic energy. All ions are given roughly the same amount of energy, so their velocity will differ depending on their mass and charge.
- Separation Based on Mass-to-Charge Ratio (m/z):
The core function of the mass analyser is to sort the ions based on their mass-to-charge ratio:
• Light ions or highly charged ions are affected more by the electric or magnetic fields and take a different path or speed.
• Heavier or singly charged ions behave differently, allowing the analyser to distinguish them.
• Types of Mass Analysers:
Each analyser has unique features, such as resolution, speed, and sensitivity, that make them suitable for different applications. Different instruments use various principles to separate ions:
• Quadrupole: Uses oscillating electric fields to filter ions. Only ions of a specific m/z can pass through at any given time. Offers good sensitivity and resolution for targeted quantification, with the ability to perform tandem MS (MS/MS) experiments.
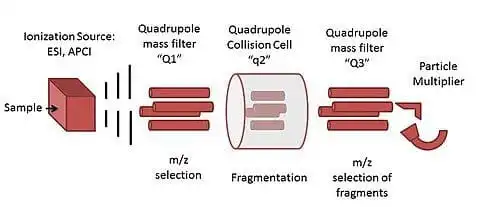
Figure 4: Quadrupole mass analyser illustrative diagram
• Time-of-Flight (TOF): Ions are accelerated down a flight tube to measure the flight time of ions through a defined distance, which directly relates to their mass to charge ratio as lighter ions reach the detector faster than heavier ones. Provides high-resolution analysis and is often used for applications requiring high accuracy in mass measurement, such as proteomics.
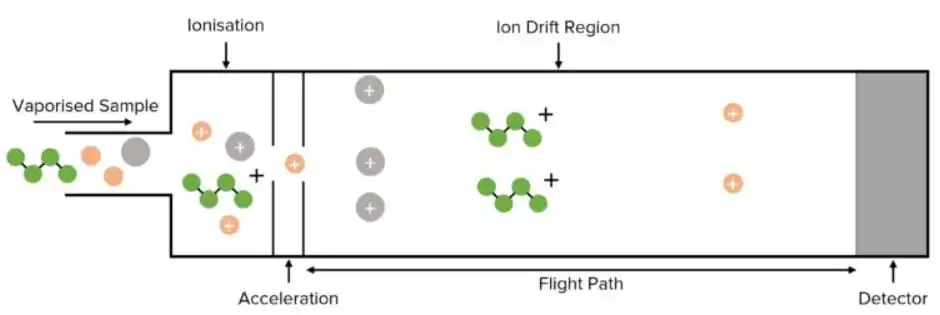
figure 5:Time of flight mass analyser diagram
• Ion Trap (or Orbitrap): Traps ions in an electric field and measures their oscillation frequency to determine m/z. Combines high resolution and high accuracy mass measurements, making it suitable for complex mixtures and high-throughput analyses.
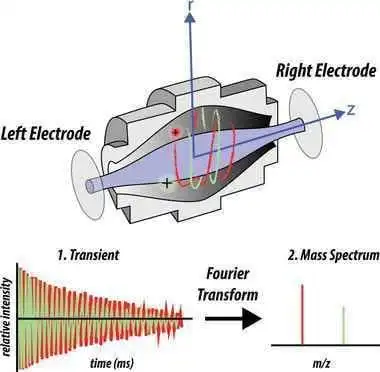
figure 6: orbitrap mass analyser
• Magnetic Sector: Bends the path of ions using a magnetic field—light ions bend more, heavy ions less.
Detection
After separation, ions strike a detector (like an electron multiplier), generating a signal proportional to the ion abundance, which is then used to determine the concentration and structure of the sample components.
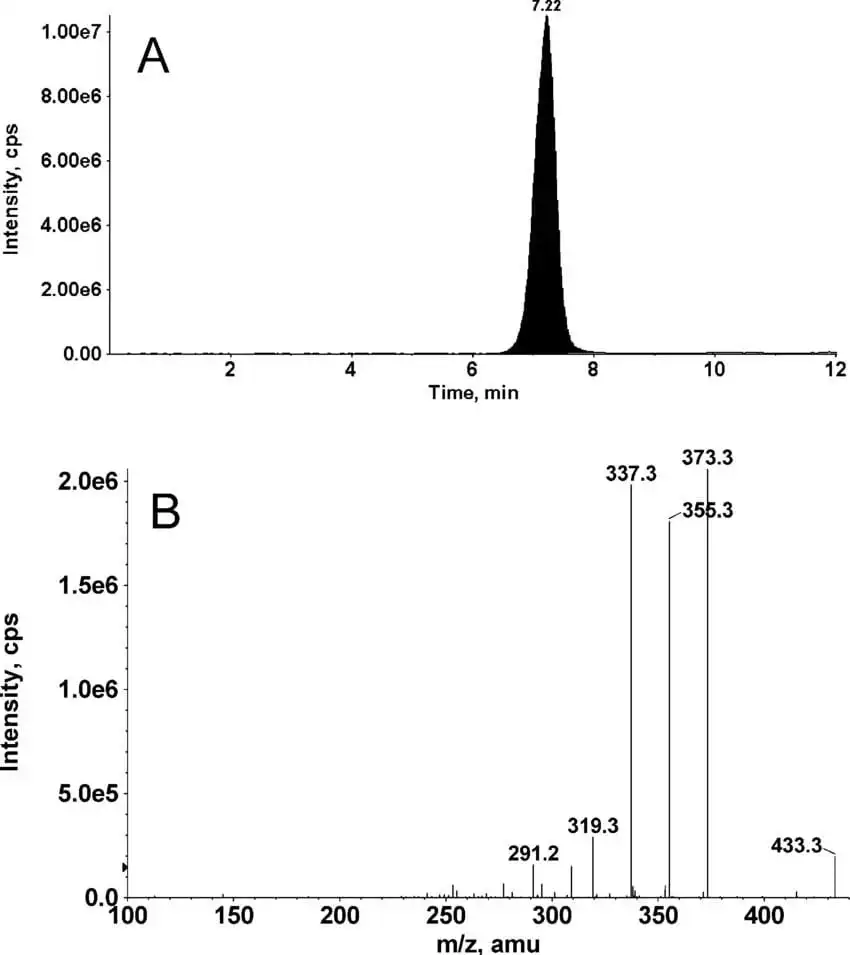
Figure 7: A) LC-MS/MS chromatogram (sum of m/z 373, 355, and 337) and (B) positive ESI-MS/MS spectrum
WHAT DOES THIS ACHIEVE?
This results in a mass spectrum: a graph with peaks representing different ions. The position of each peak corresponds to the mass-to-charge ratio, and the height (intensity) shows how much of that ion was present.
This allows scientists to:
• Identify unknown compounds
• Determine molecular weights
• Analyse mixtures
• Detect trace amounts of substances
Applications of LC-MS
Pharmaceutical and Biotechnology
LC-MS is extensively used in the pharmaceutical industry for the analysis of drugs and their metabolites. It plays a pivotal role in pharmacokinetic studies, where it is used to measure the concentration of drugs in biological samples (e.g., plasma, urine). LC-MS is also used for:
• Bioavailability and bioequivalence studies: Measuring the absorption and distribution of drugs.
• Drug metabolism and pharmacogenetics: Studying the breakdown of drugs in the body and identifying genetic factors influencing drug metabolism.
• Peptide and protein analysis: Characterizing therapeutic proteins and biomarkers in drug development.
Clinical Diagnostics
In clinical diagnostics, LC-MS is used to detect and quantify a wide range of biomarkers, metabolites, and small molecules in biological fluids. It is invaluable in:
• Toxicology: Monitoring drug abuse, poisons, and other toxins in blood and urine.
• Endocrinology: Analysing hormones and metabolic disorders.
• Proteomics: Profiling proteins to identify biomarkers for diseases such as cancer.
Environmental Monitoring
LC-MS is a powerful tool in environmental analysis, where it is used to detect contaminants such as pesticides, pharmaceuticals, and industrial chemicals in water, soil, and air samples. The high sensitivity and selectivity of LC-MS make it ideal for trace analysis of environmental pollutants.
Food and Agriculture
In the food industry, LC-MS is employed to analyse food contaminants, such as pesticides, preservatives, and additives. It is also used for the quality control of food products and to monitor food safety regulations.
Forensic Science
LC-MS is a key tool in forensic toxicology, where it is used for the identification and quantification of drugs and poisons in biological samples. Its sensitivity enables the detection of trace amounts of substances, making it essential in criminal investigations and post-mortem toxicological studies.
Challenges and Limitations of LC-MS
While LC-MS offers numerous advantages, it also has several challenges:
• Sample Matrix Interference: Biological samples often contain a complex mixture of proteins, lipids, and other substances that can interfere with the analysis. Sample preparation techniques such as solid-phase extraction (SPE) or protein precipitation are often employed to minimize these interferences.
• Ionization Efficiency: Some compounds do not ionize efficiently, which can lead to poor sensitivity. The choice of ionization technique and optimization of experimental conditions is crucial for maximizing sensitivity.
• Instrumental Cost: LC-MS systems are expensive, and maintaining and calibrating the equipment can incur high operational costs.
• Data Analysis: The large volume of data generated by LC-MS requires advanced software for processing and interpretation. Proper data analysis is crucial for accurate identification and quantification of analytes.
Future Directions in LC-MS: Where Innovation Meets Precision
The future of Liquid Chromatography-Mass Spectrometry (LC-MS) is paved with exciting advancements that promise to push the boundaries of analytical science. As demand grows for more sensitive, accurate, and versatile analytical tools, LC-MS continues to evolve. Breakthroughs in high-resolution and high-accuracy mass spectrometry are unlocking new possibilities in the analysis of complex biological samples, from proteomics to metabolomics. At the same time, the miniaturization of LC-MS systems is opening doors for portable, point-of-care testing in clinical diagnostics and real-time environmental monitoring. Innovations in ionization techniques and liquid chromatography methods are further refining the power of LC-MS, making it faster, more efficient, and more accessible than ever before. With these advancements, LC-MS is not just keeping pace with scientific demand — it’s leading the way into the next era of precision analysis.
Conclusion
Liquid Chromatography-Mass Spectrometry (LC-MS) stands as a powerhouse in modern analytical chemistry — a technique that’s not just versatile, but essential. Whether it’s unlocking new drug discoveries, diagnosing diseases with precision, tracking environmental pollutants, or advancing forensic investigations, LC-MS delivers unmatched sensitivity, accuracy, and insight. As technology evolves, so too does LC-MS — with cutting-edge improvements in instrumentation, ionization methods, and data analysis tools pushing the boundaries of what’s possible. As a result, LC-MS will remain at the forefront of innovation, driving discoveries and setting new standards in analytical science for years to come.
References
- Lough, W. (2020). Liquid Chromatography–Mass Spectrometry: Applications in Biological Analysis. Wiley.
- Mann, M., & Kulak, N. A. (2021). “The New Era of Mass Spectrometry.” Trends in Analytical Chemistry, 131, 115975
- Evers, R. A., & Goodwin, J. P. (2019). Pharmaceutical Applications of Liquid Chromatography–Mass Spectrometry. Elsevier.
- Nguyen, T. M., & Jafari, M. (2018). “Recent Advances in Liquid Chromatography-Mass Spectrometry: Applications to Clinical Diagnostics.” Clinical Chemistry, 64(5), 737-749.
- McLafferty, F. W., & Tureček, F. (1993). Interpretation of mass spectra (4th ed.). University Science Books.
- de Hoffmann, E., & Stroobant, V. (2007). Mass spectrometry: Principles and applications (3rd ed.). Wiley.
- Sparkman, O. D., Penton, Z. E., & Kitson, F. G. (2011). Gas chromatography and mass spectrometry: A practical guide (2nd ed.). Academic Press.
- Harris, D. C. (2015). Quantitative chemical analysis (9th ed.). W. H. Freeman and Company.
- National Centre for Biotechnology Information. (n.d.). PubChem and literature database. U.S. National Library of Medicine. https://www.ncbi.nlm.nih.gov
- Agilent Technologies. (n.d.). Basics of LC/MS – a primer. Agilent Technologies. Retrieved June 14, 2025, from https://www.agilent.com/en/product/liquid-chromatography-mass-spectrometry-lc-ms/lcms-fundamentals
- Science Info. (2023, June). LC‑MS: Definition, instrumentation, applications. Science Info. Retrieved June 14, 2025, from https://scienceinfo.com/lc-ms-instrumentation-applications/
- ScienceDirect. (n.d.). High-performance liquid chromatography. ScienceDirect. Retrieved June 17, 2025, from https://www.sciencedirect.com/topics/engineering/high-performance-liquid-chromatography
- Lab Training. (n.d.). How LC-MS/MS scores over LC-MS. Lab Training. Retrieved June 17, 2025, from https://lab-training.com/how-lc-msms-scores-over-lc-ms/?amp
- B-A-C. (n.d.). Types of mass analyzers: Time of flight (TOF). B-A-C. Retrieved June 17, 2025, from https://b-ac.co.uk/types-of-mass-analysers-time-of-flight-tof/
- Savaryn, John & Toby, Timothy & Kelleher, Neil. (2016). A researcher’s guide to mass spectrometry‐based proteomics. PROTEOMICS. 16. 10.1002/pmic.201600113.
- Tor, Elizabeth & Filigenzi, Michael & Puschner, Birgit. (2005). Determination of Oleandrin in Tissues and Biological Fluids by Liquid Chromatography−Electrospray Tandem Mass Spectrometry. Journal of agricultural and food chemistry. 53. 4322-5. 10.1021/jf050201s.


