1. Introduction
The development of effective oral pharmaceutical formulations for poorly water-soluble drugs remains one of the most critical challenges in drug delivery science. Oral administration is favored for its convenience, safety, and patient compliance, but the bioavailability of active pharmaceutical ingredients (APIs) administered via this route is often limited by their physicochemical properties, particularly aqueous solubility and membrane permeability.
According to the Biopharmaceutics Classification System (BCS), drugs are categorized based on their solubility and permeability characteristics. BCS Class II (low solubility, high permeability) and Class IV (low solubility, low permeability) drugs, in particular, present significant formulation challenges due to their poor dissolution profiles in gastrointestinal (GI) fluids, which often result in erratic or suboptimal systemic absorption.
To address these challenges, formulation scientists have turned to functional excipients—substances added to pharmaceutical formulations not only to provide bulk or facilitate manufacturing but also to directly enhance the dissolution, solubility, and bioavailability of the API. The integration of such excipients into solid oral dosage forms allows for modulation of key parameters such as wetting, disintegration, micelle formation, pH microenvironment, and even molecular dispersion.
This article provides a detailed review of the mechanisms and applications of various functional excipients used in modern solid oral dosage forms. Emphasis is placed on scientific rationale, in vitro and in vivo performance data, and real-world case examples.
Classification of Pharmaceutical Excipients
Pharmaceutical excipients are often present in larger quantities than the active pharmaceutical ingredient (API), sometimes constituting up to 90% of a dosage form’s total mass or volume. Their functions go beyond mere bulking—they directly influence drug stability, release, and patient safety.
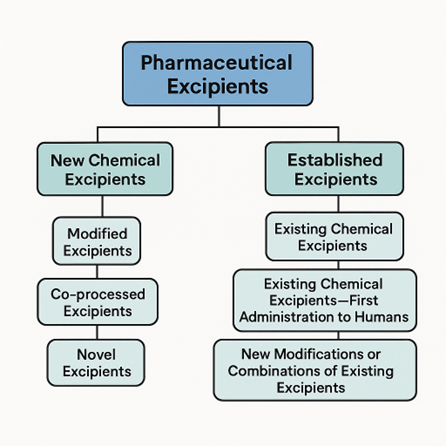
To help evaluate and regulate these substances, the International Pharmaceutical Excipient Council (IPEC) has developed a detailed classification system. This system groups excipients based on their chemical nature and safety data.

- Main Categories
Excipients fall into two broad classes:
- Established Excipients
- New Chemical Excipients
Each class includes several subcategories with specific regulatory and safety requirements.
- Established Excipients
These are excipients with a history of safe use in pharmaceutical products. IPEC subdivides them into three groups:
- Existing Chemical Excipients
These are excipients that have been previously used in approved formulations without modification. - Existing Chemical Excipients — First Administration to Humans
These are used for the first time in a human drug product, despite being chemically known. - New Modifications or Combinations of Existing Excipients
These include changes in formulation, such as different blends or alterations that do not create a new chemical entity but affect performance or safety.
- New Chemical Excipients
These excipients are either completely new to pharmaceutical use or chemically altered forms of known substances. They are classified into:
- Modified Excipients
These are existing excipients with changes in purity, particle size, or physical characteristics. The chemical structure remains unchanged, but functional properties may differ. - Co-processed Excipients
These are combinations of two or more established excipients processed together—often via spray drying or granulation—to create a new excipient with improved functionality. These properties cannot be achieved by simply mixing the individual excipients. - Novel Excipients
These are entirely new chemical entities used for the first time in pharmaceutical products. This category may also include chemically modified versions of existing excipients, especially if the change results in new functionality or safety concerns.
The need for strict classification and evaluation is underscored by historical safety incidents. For example, in 2008, the inclusion of glycerin contaminated with diethylene glycol in pediatric teething powder led to the deaths of 84 children. This tragedy highlighted the critical need for stringent safety and quality control of excipients, especially for vulnerable populations.
As a result, global regulatory bodies have implemented more rigorous and dynamic guidelines for excipient use and safety assessment.
2. Overview of Functional Excipients
Tackling Poor Aqueous Solubility in Modern Drug Development
One of the most persistent challenges in pharmaceutical formulation is poor aqueous solubility. Around 40% of medications currently available in pharmacies have low water solubility. The situation is even more critical in drug development, where nearly 90% of new chemical entities (NCEs) in the pipeline exhibit the same limitation.
Why Solubility Matters
Solubility directly impacts a drug’s bioavailability, which is the fraction of the drug that reaches systemic circulation and produces a therapeutic effect. According to the Biopharmaceutics Classification System (BCS), two key properties determine bioavailability:
- Aqueous solubility
- Membrane permeability
A drug with low solubility (typically <0.1 mg/mL) dissolves slowly in the gastrointestinal fluids. As a result, its absorption is limited by the dissolution rate, especially for BCS Class II and Class IV drugs.
Role of Functional and Specialty Excipients
To address this problem, functional excipients are added to formulations. These ingredients do more than improve stability or manufacturability—they enhance drug performance in critical ways:
- Improve solubility and dissolution
- Modify the release rate (e.g., sustained or controlled release)
- Enable targeted delivery to specific regions in the gastrointestinal tract
- Re-formulate older drugs into more effective and cost-efficient alternatives
Specialty excipients fall into this category. They are carefully selected to optimize drug release rate, location, and efficiency.
Strategies to Enhance Solubility
Several physico-chemical and formulation-based techniques have been explored to improve the solubility of poorly soluble drugs:
Physico-chemical techniques:
- Pro-drug formation (converts the drug into a more soluble derivative)
- Salt formation (creates ionic versions of the drug)
- Co-precipitation and solvent evaporation
- Micronization (reduce particle size to increase surface area)
Formulation strategies:
- Hot-melt extrusion or melt granulation
- Solid dispersions
- Inclusion complexes (e.g., cyclodextrin-based systems)
In addition, excipients such as:
- Surfactants (to lower surface tension),
- Polymers (to stabilize amorphous forms),
- Super-disintegrants
They are used to boost the apparent solubility and promote better drug absorption.
These excipients do not merely play a supportive role but are often critical to the success of the dosage form in achieving adequate bioavailability.
I. Inclusion Complexes: Cyclodextrins
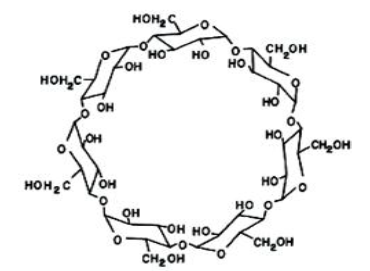
Cyclodextrins (CDs) are cyclic oligosaccharides composed of 6 (α-CD), 7 (β-CD), or 8 (γ-CD) glucose units. Their unique molecular architecture, featuring a hydrophilic outer surface and a lipophilic inner cavity, enables the formation of host–guest inclusion complexes with hydrophobic drug molecules. This inclusion mechanism leads to an increase in the apparent solubility of poorly soluble APIs.
The main properties of these Cyclodextrins are given in Table 1.

Among all cyclodextrins, β-cyclodextrin (β-CD) is the most widely available, the least expensive, and generally the most versatile.
While cyclodextrins are considered safe for oral administration, β-CD can cause kidney toxicity (nephrotoxicity) when given parenterally (e.g., by injection).
To address this issue, safer derivatives like hydroxypropyl-β-cyclodextrin (HP-β-CD) and sulfobutyl ether-β-cyclodextrin (SBE-β-CD) have been developed.
For example, carbamazepine, a BCS Class II drug, has shown remarkable improvement in solubility and bioavailability when complexed with hydroxypropyl-β-cyclodextrin (HP-β-CD). In studies, a formulation containing carbamazepine and HP-β-CD with hydroxypropyl methylcellulose (HPMC) increased the drug’s solubility by 95-fold and improved bioavailability in beagle dogs by 1.5-fold compared to a commercial product.
Although cyclodextrins are generally safe for oral use, β-CD is nephrotoxic when administered parenterally, prompting the development of safer derivatives such as HP-β-CD and sulfobutyl ether β-cyclodextrin (SBE-β-CD).
II. Superdisintegrants
Before a tablet can deliver its intended effect, it needs to break apart, or disintegrate, after swallowing. This step is essential. Without disintegration, the active pharmaceutical ingredient (API) won’t dissolve properly, and if it doesn’t dissolve, it won’t be absorbed into the body.
This is where disintegrants come in.
Disintegrants are inactive ingredients added to tablet formulations to help them fall apart quickly in the gastrointestinal tract. Their main job is to increase the surface area of the tablet by breaking it into smaller pieces. This allows water to reach the API faster, speeding up dissolution and improving bioavailability (how much drug gets into the bloodstream).
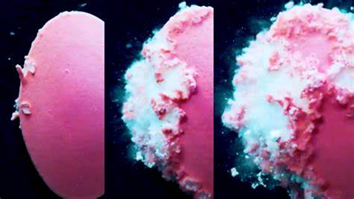
How Disintegrants Work
Disintegrants work through different mechanisms. The most common are:
- Swelling: The disintegrant absorbs water and expands, pushing particles apart.
- Wicking (capillary action): Water is pulled into the tablet through small pores, breaking bonds between particles.
- Particle repulsion: Once hydrated, particles may repel each other, weakening the tablet structure.
- Deformation recovery: Some materials are compressed during tableting, then bounce back when wet forcing the tablet to break apart.
Among these, swelling and wicking are the most dominant.
To be effective, disintegrants need space to work. The tablet must be porous enough to let water in. If the tablet is too dense, water won’t penetrate, and the disintegrant can’t function.
Example: Valsartan and Superdisintegrants
In a study focused on valsartan—a poorly soluble drug used to treat high blood pressure—researchers tested fast disintegrating tablets using sodium starch glycolate and crospovidone at different concentrations.
Results were clear:
- At 5% w/w, both disintegrants helped the tablets disintegrate in under 2 minutes.
- These tablets released the drug faster and in greater amounts than a standard suspension.
- Crospovidone lowered blood pressure by 60 mm Hg and sodium starch glycolate by 20 mm Hg within 1 hour.
- In comparison, the reference suspension only dropped blood pressure by 10 mm Hg over 3 hours.
- Fast disintegration meant the drug started working within 15 minutes, while the suspension took an hour.
This shows that faster disintegration doesn’t just improve drug absorption but can also directly affect how quickly the medicine works in the body.
III. pH-Modifying Agents
Why Local pH Near Drug Particles Matters
When a tablet dissolves, each drug particle is surrounded by a thin fluid layer, the stagnant diffusion layer. Its pH can differ from the surrounding GI fluid and strongly affects how well weak acids or bases dissolve.
Many drugs dissolve better when ionized. This depends on the local pH. To improve this, pH-adjusting excipients are added to the formulation. When the dosage form breaks apart, these excipients dissolve and release H⁺ or OH⁻ ions, adjusting the pH around the drug particle.
This local shift in pH promotes ionization and faster dissolution, especially for poorly soluble drugs.
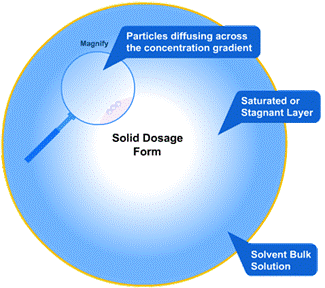
The goal is simple: tweak the local pH right at the surface of the drug particles to favour ionization and therefore enhance dissolution. For example: A weak acid (like ibuprofen) dissolves better in a slightly basic environment, so a basic excipient is added to raise the local pH.
Common pH modifiers
- Acids: Citric acid, tartaric acid, carbonic acid
- Alkalinizing agents: Sodium bicarbonate, calcium carbonate, sodium carbonate
Example: Inclusion of sodium bicarbonate in paracetamol tablets reduced Tmax and increased Cmax in human subjects, indicating faster absorption.
IV.Amorphous Solid Dispersions (ASDs)
Solid dispersions represent a class of advanced formulation strategies wherein poorly soluble drugs are molecularly dispersed within a hydrophilic polymer matrix. These systems often present the drug in an amorphous, higher-energy state, which significantly improves its apparent solubility and dissolution rate. ASDs are typically produced via spray drying, hot melt extrusion, freeze drying, or solvent evaporation.
Hydrophilic polymers employed in ASDs include:
- Polyvinylpyrrolidone (PVP)
- Hydroxypropyl methylcellulose (HPMC)
- Carboxymethylcellulose (CMC)
You can read more about spray dryer and solid dispersion: Optimizing Drug Solubility: The Role of Spray Drying in Pharmaceutical Formulation
Case studies:
- Nilotinib (BCS class II) exhibited low and variable bioavailability (~30%). An ASD prepared with Soluplus® using spray drying improved solubility substantially.
- Though vivo data are pending, this shows potential for therapeutic advancement.
- Posaconazole, a lipophilic antifungal, was dispersed in HPMC acetate succinate (HPMC-AS) using hot melt extrusion. The resultant formulation demonstrated supersaturation in GI fluids, with improved absorption even in fasted states, unlike the commercial oral suspension that required co-administration with food.
ASDs thus represent one of the most versatile and effective platforms for enhancing drug solubility and bioavailability.
V. Surfactants
For a drug to be absorbed after oral administration, it must first dissolve in gastrointestinal fluids. Many active pharmaceutical ingredients (APIs), especially those that are poorly water-soluble, struggle at this stage. Surfactants play a critical role in overcoming this limitation.
What Are Surfactants?
Surfactants are amphiphilic molecules—they contain both hydrophilic (water-attracting) and hydrophobic (water-repelling) groups. This dual nature allows them to position themselves at interfaces, such as between the solid API and the aqueous medium.
Once the critical micelle concentration (CMC) is reached, surfactants self-assemble into micelles. These micelles have hydrophobic cores that can solubilize poorly water-soluble drugs, enhancing their apparent solubility and dissolution rate.
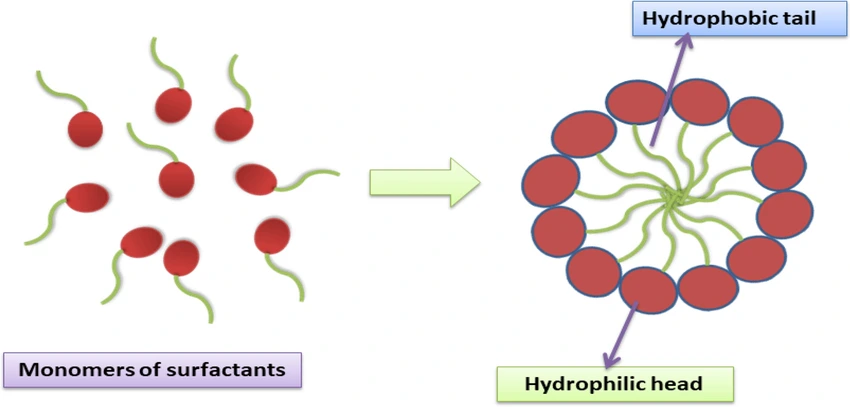
Why This Matters in Tablets
Many tablet formulations include APIs and excipients that do not wet easily. This poor wettability slows down disintegration and dissolution, reducing bioavailability. Surfactants reduce interfacial tension, improve wetting of the API surface, and can accelerate drug release.
Commonly used surfactants in solid dosage forms include:
- Sodium lauryl sulfate (SLS) – Anionic, cost-effective, widely used
- Poloxamers – Non-ionic block copolymers, good solubilizers
- Tween® series (20, 40, 60, 80) – Non-ionic, biocompatible, often used in poorly soluble APIs
Examples from Literature
- SLS at 5% significantly improved celecoxib dissolution up to a 3-fold increase in just 20 minutes.
- Tween® 80, due to a double bond in its hydrophobic chain, achieved the highest solubility enhancement for ibuprofen among all surfactants tested in one study.
3. Conclusion
The formulation of solid oral dosage forms for poorly soluble APIs necessitates the integration of excipients that go beyond traditional roles. Functional excipients—whether they function by altering the drug’s physical state, modifying the local dissolution environment, or promoting solubilization through micellar or emulsified systems—are central to improving therapeutic performance.
The studies summarized in this review consistently show that functional excipients:
- Improve dissolution rates dramatically
- Enhance bioavailability in both preclinical and clinical models
- Enable the reduction of dose without compromising efficacy
- Support the development of fast-acting, stable, and patient-friendly dosage forms
However, further research is warranted to:
- Generalize findings across different API classes
- Expand in vivo and clinical studies
- Evaluate long-term stability and patient outcomes
- Address regulatory considerations for novel excipients
References: