What is a pharmaceutical excipient?
A Pharmaceutical excipient is an inactive element, distinct from the active pharmaceutical ingredient(s), used in manufacturing a pharmaceutical product to improve its function. Carrying on with Pharmaceutical Excipient (Episode-01), which describes excipients used in solid dosage forms. Pharmaceutical Excipients: The Unsung Heroes of Pharmaceutical Formulations (Episode 02—Final Part) mainly focuses on describing the excipients used in Semisolid Dosage Form, Liquid Dosage Form, and parenteral dosage form.a
Surfactants
It is composed of polar and non-polar regions. Surfactants are molecules that can cluster in solution and create micelles where non-polar medications can be incorporated and solubilized. They can lower the surface tension or interfacial tension between a liquid and a solid, a gas and a liquid, or among two liquids.
Mechanism
Surfactants in pharmaceutical formulations have various important processes by which they influence things.
Lowering Surface and Interfacial Tension
Surfactant molecules go to the interface separating two phases. While their hydrophilic heads engage with the polar phase, like water, their hydrophobic tails point toward the non-polar phase, such as oil or air. This configuration disturbs the intermolecular interactions at the contact, therefore greatly lowering surface tension (at a liquid-gas interface) or interfacial tension (at a liquid-liquid or liquid-solid interface).
Wetting
Surfactants increase the capacity of the liquid to spread over the solid by lowering the contact angle between a liquid and a solid surface. Water must do this if it is to enter the hydrophobic pores of a tablet.
Solubilization/ Dissolving
Above a particular concentration known as the critical micelle concentration (CMC), surfactant molecules in an aqueous environment self-assemble into spherical structures known as micelles. These micelles feature a hydrophilic outer shell (made by the heads) and a hydrophobic center (produced by the tails). Drugs with poor water solubility can be divided into the micelles’ hydrophobic core, hence boosting their apparent solubility in the aqueous environment.
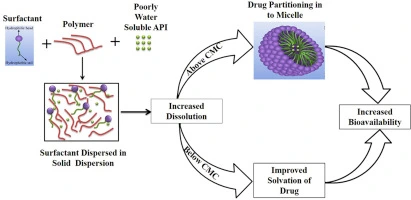
Types & Example
Surfactants are classified based on the charge of their hydrophilic head group when dissolved in water. The four main types are:
1. Anionic Surfactants: Negatively charged hydrophilic head.
Examples: Sodium Lauryl Sulfate (SLS), Sodium Laureth Sulfate (SLES), Soaps (sodium or potassium salts of fatty acids, e.g., Sodium Stearate), Alkylbenzene Sulfonates (ABS), Linear Alkylbenzene Sulfonates (LAS), & Docusate Sodium (Dioctyl Sodium Sulfosuccinate)
2. Cationic Surfactants: Positively charged hydrophilic head.
Examples: Quaternary Ammonium Compounds (Quats) such as: Cetrimonium Bromide (CTAB), Benzalkonium Chloride (BKC), Behentrimonium Chloride, Amine salts (at acidic pH)
3. Nonionic Surfactants: Nonionic surfactants have no net charge on the hydrophilic head. Polar groups such as ether or hydroxyl cause their hydrophilic character.
Examples: Alcohol Ethoxylates (e.g., Laureth-3, Laureth-23, Steareth-20), Alkylphenol Ethoxylates (APEs) (e.g., Nonylphenol Ethoxylates), Fatty Acid Esters (e.g., Sorbitan Esters like Sorbitan Monolaurate (Span 20), Sorbitan Monopalmitate (Span 40), Sorbitan Monostearate (Span 60), Sorbitan Monooleate (Span 80), Polysorbate 20, Polysorbate 40, Polysorbate 60, Polysorbate 80).
4. Amphoteric (Zwitterionic) Surfactants: Have on the hydrophilic head both a positive and a negative charge. The pH of the solution determines the net charge.
Examples: Lecithin, Amino Oxides (e.g., Lauramine Oxide), Sulfobetaines (Sultaines), Betaines (e.g., Cocamidopropyl Betaine)
Release modifying agents
Substances called release-modifying agents are excipients used to regulate the release of drugs in modified-release formulations like controlled-release or extended-release tablets. As excipients in tablets with modified release, they play a vital role.
Example:
Carbomer Copolymer, Shellac, Carbomer 934P, 980P & 971P, Hypromellose, Carboxymethylcellulose Sodium, Ethyl cellulose, Starch Pregelatinized Modified, Glyceryl Monostearate, Guar Gum, Hydroxypropyl Cellulose, Polyethylene Oxide, Polyvinyl Acetate Dispersion, Xanthan Gum, Alginic Acid.
Preservatives
Preservatives are often incorporated in formulations to prevent microbial contamination, also to increase their shelf life. These preservative solutions guarantee that the performance of the product stays intact and protect it from microbiological growth.
Example:
Benzoic acid & its salts, Sorbic acid and its salts, Methyl paraben, Ethyl parabens, Propyl paraben, Benzyl Alcohol, Benzalkonium Chloride, Phenol, Thiomersal.
Antioxidants
Pharmaceutical antioxidants are chemicals added to medicine formulations to prevent or delay the oxidation of excipients and active pharmaceutical ingredients (APIs). A chemical process called oxidation can harm these components and cause a loss of efficacy, color changes, the generation of undesired byproducts, and a general reduction in the shelf life of the drug. Antioxidants work either by simply oxidizing themselves or by interfering with the free radical chain events, pushing oxidation ahead.
Example:
Sodium Metabisulfite, Alpha-Tocopherol (Vitamin E), Sodium Sulfite, Monothioglycerol (Thioglycerol), Butylated Hydroxyanisole (BHA), Butylated Hydroxytoluene (BHT), Ascorbic acid.
Buffering agents
Pharmaceutical buffering agents are chemicals or mixtures of chemicals meant to modify pH changes caused by the introduction of small amounts of acids or bases or during dilution. The stability, solubility, efficacy, and patient comfort of a pharmaceutical product depend on its pH being maintained within a specified range. Usually, buffers are composed of a weak acid coupled with its conjugate base or a weak base mixed with its conjugate acid; these two components work together to neutralize extra hydrogen or hydroxide ions.
Example:
Phosphate Buffers: Sodium Dihydrogen Phosphate (NaH2PO4) and Disodium Hydrogen Phosphate Na2HPO4). They provide buffering capacity across a broad pH range, roughly between pH 5.8 and 8.0, depending on the specific salts and their concentrations.
Acetate Buffers: Acetic acid, a weak acid, and its corresponding salt, Sodium acetate. Acetate buffers are effective within a slightly acidic to nearly neutral pH range, approximately pH 3.6 to 5.6.
Citrate Buffers: Citric acid and one of its salts, Sodium citrate. They provide buffering capacity over a wider range due to their multiple pKa values, around pH 3.0 to 6.2.
Borate Buffers: Boric acid (H3BO3) and its salts, Sodium borate (Na2B4O7). These buffers are generally applied in slightly alkaline conditions, with a pH around 8.0 to 10.0, and are commonly found in ophthalmic solutions.
Co-solvents
Pharmaceutical co-solvents are organic solvents that blend effectively with water and are used in aqueous drug formulations to increase the solubility of poorly soluble drugs. These solvents alter the solvent system’s polarity, reduce the solute’s intermolecular forces, and/or improve the interaction between the solute and the solvent combination. Co-solvents can improve drug dissolution, absorption, and overall bioavailability.
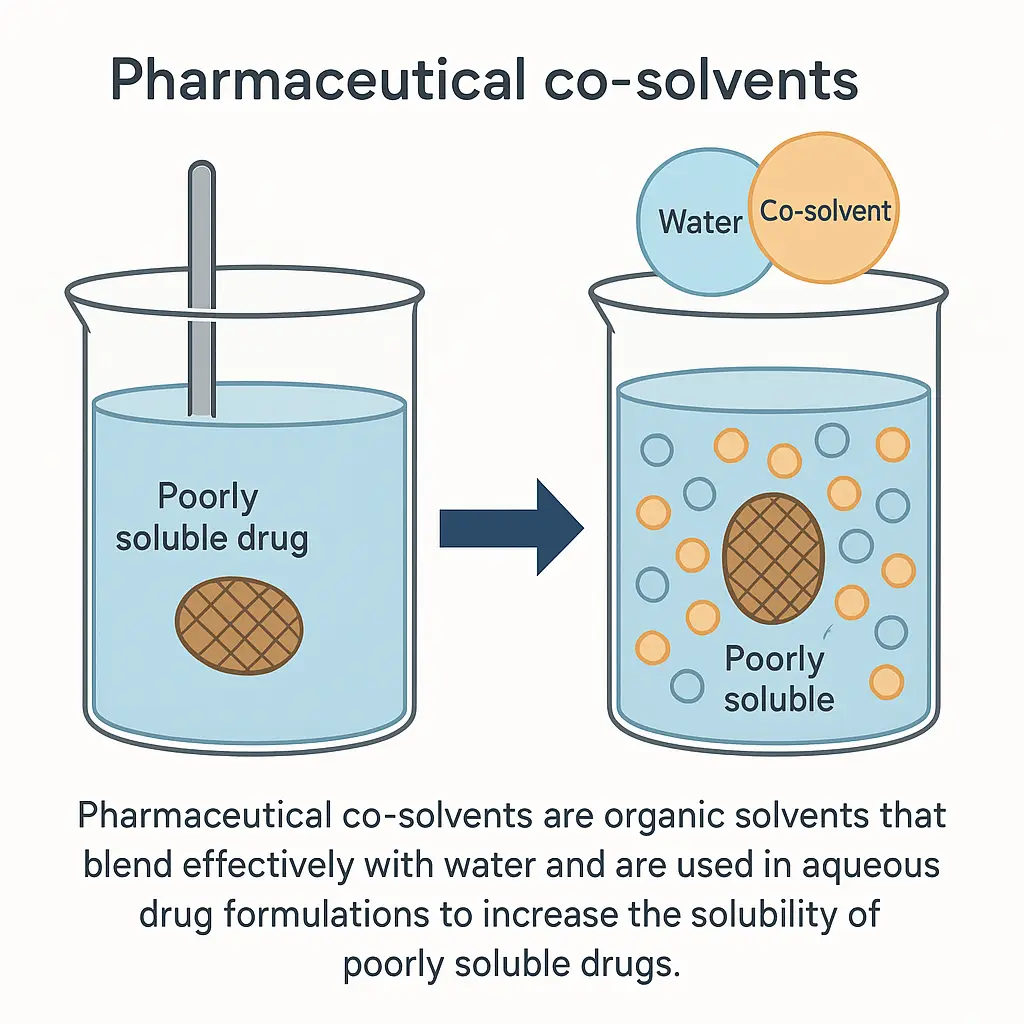
Example: Ethanol, Propylene Glycol, Polyethylene Glycol, Glycerol, Sorbitol Solution, N-Methyl-2-pyrrolidone & DMSO
Viscosity Imparting Agents
Commonly known as viscosifiers, thickening agents, or suspending agents, viscosity-imparting substances in pharmaceuticals are excipients used in liquid and semi-solid medication formulations to increase their viscosity.
Example:
Carbomers (Carbopol), Xanthan Gum, Carboxymethylcellulose Sodium (CMC-Na), Hydroxypropyl Methylcellulose (HPMC), Methylcellulose (MC).
Humectants
Moisture-attracting pharmaceutical humectants are included in topical and oral drug formulations to help preserve moisture levels. They work by either moving water from the dermis to the stratum corneum, the outer layer of the skin, or by sucking in and holding water from the surrounding atmosphere. This activity improves the product’s general texture and look, helps prevent dryness, maintains skin moisture, and increases skin elasticity. In oral formulations, they can also assist in preventing the drying out of the product.
Example:
Propylene Glycol, Polyethylene Glycol, Glycerol & Sorbitol Solution
Chelating Agents
Chemical compounds known as chelating agents can form stable, water-soluble complexes with metal ions. “Chelete” is from the Greek word “chele,” which means “claw,” so showing how these agents “grasp” and bind to metal ions using many coordination bonds, producing a ring-like structure. Known as chelation, this process effectively sequesters the metal ion.
Example:
Dimercaprol (BAL – British Anti-Lewisite), Edetate Calcium Disodium, Citric Acid, & Ethylenediaminetetraacetic acid (EDTA).
Emulsifying Agents (Emulsifiers)
Emulgents, or emulsifying agents, are substances that stabilize emulsions by reducing the interfacial tension between incompatible liquids, usually oil and water, and forming a protective barrier around the dispersed droplets. This process produces a stable and homogenous mixture by preventing the droplets from combining and splitting into different layers.
The amphiphilic property of them sits at the border between the two phases, so improving their mixing and avoiding separation. In pharmaceutical goods, emulsifying agents are crucial for creating stable emulsions like creams, lotions, and some injectable solutions (e.g., lipid emulsions used for intravenous feeding). They ensure regular distribution of the active pharmaceutical ingredient (API) and help to shape the texture, appearance, and stability of the product.
Example:
Glyceryl Monostearate & Carbomers (934P, 971P & 980P), Cetyl Alcohol and Stearyl Alcohol, Polysorbate 20, 40, 60, 80, Sorbitan Monooleate, Sorbitan Monostearate (Span 60), Acacia, Tragacanth, Lecithin.
Suspending Agents
Excipients included in liquid medicinal products, suspending agents, are meant to increase the viscosity of the medium and lower the sedimentation rate of undissolved solid particles suspended inside it. These agents help to maintain a constant distribution of the drug across the liquid, so guaranteeing that the patient gets a correct and consistent dose with every administration. Suspensions are thermodynamically unstable systems; suspending agents are crucial for enhancing their physical stability, employing caking prevention (the creation of a compact sediment difficult to redisperse) and simple redispersion upon shaking.
Example:
Methylcellulose (MC), Hydroxypropyl Methylcellulose (HPMC), Carboxymethylcellulose Sodium (CMC-Na), Xanthan Gum & Carbomers (934P, 971P & 980P).
Emollients
Topical emollients are ingredients meant to calm and moisturise the skin. By filling the gaps between dead skin cells with lipids and other substances, they produce a more uniform and flexible skin texture. By creating a protective barrier on the surface, emollients also help to prevent water loss from the stratum corneum, the topmost layer of skin. This hydrates the skin and addresses issues including dryness, flaking, roughness, and irritation. Often found in creams, lotions, ointments, and moisturizers, they help to enhance skin texture and effectiveness.
Example:
Petrolatum (Vaseline), Stearic Acid & Lanolin, Dimethicone, Isopropyl Myristate, Cetyl Alcohol, Sepineo P600, Liquid paraffin.
Semisolid Bases
Semisolid bases act as the main medium in semisolid dosage forms like ointments, creams, gels, and pastes. These bases are composed of a mixture of materials that retain a supple and pliable consistency at room temperature, intended for use on the skin or mucous membranes. The base serves not only as a vehicle for the active pharmaceutical ingredient (API) but also influences the drug’s release, absorption, and the overall feel and therapeutic effects of the formulation.
Example:
Oleaginous Bases (Hydrocarbon Bases): Petrolatum (Vaseline), White Petrolatum, Yellow Ointment, Mineral Oil, Paraffin, Lanolin (anhydrous).
Absorption Bases: Hydrophilic Petrolatum, Anhydrous Lanolin. Polyethylene glycols (PEGs)
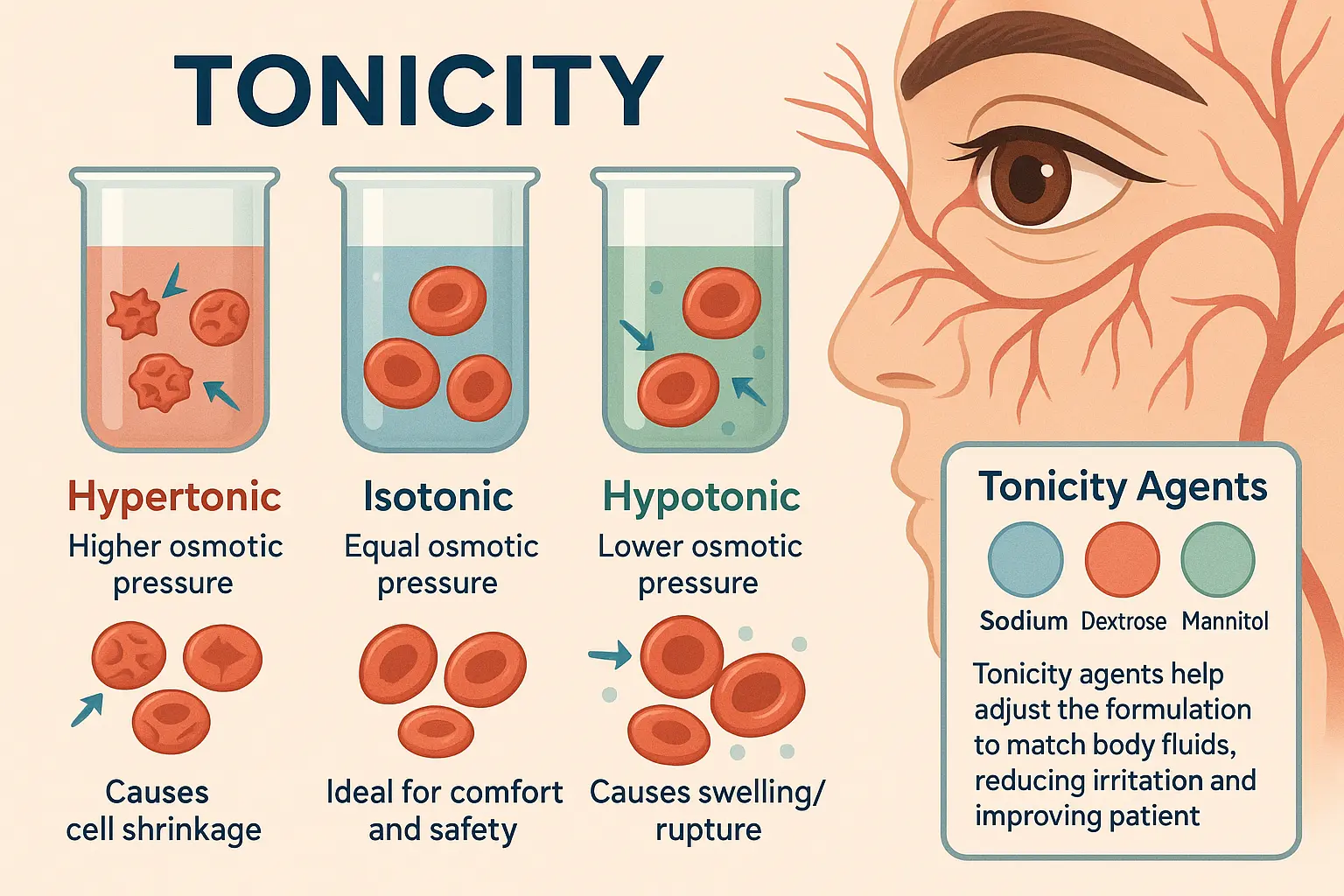
Tonicity Agents
Tonicity agents are ingredients used to change the osmotic pressure of the product to fit that of human fluids (mostly blood and tears). Isotonic is a term for solutions that match the osmotic pressure of biological fluids. Called hypertonic, a solution with higher osmotic pressure than bodily fluids draws water from cells, hence possibly causing cell shrinkage (crenation). On the other hand, a solution with lower osmotic pressure is considered hypotonic and would cause water to enter cells, hence likely causing swelling and maybe cell rupture (hemolysis in red blood cells). Tonicity compounds are crucial for guaranteeing that the formulation is isotonic or almost so, hence enhancing patient comfort and lowering negative effects.
Example:
Sodium Chloride (NaCl), Dextrose (Glucose), Mannitol & Potassium Chloride (KCl).
Conclusion
By ensuring the stability, bioavailability, safety, and efficacy of drugs, pharmaceutical excipients are absolutely vital in drug formulation. Though formerly regarded as inert, modern excipients serve vital functions affecting medication release, increasing patient compliance, and improving manufacturability. Factors like their compatibility with the active pharmaceutical ingredient (API), the planned route of medication distribution, and legal criteria influence the choice of suitable excipients.
The deliberate selection and assessment of excipients are vital. The need for new and multifunctional excipients also increases as our knowledge of their probable effects on patient well-being and therapeutic efficacy expands. Future pharmaceutical excipients concentrate on creating materials that solve difficult drug delivery problems, enable individualized therapy, and finally improve therapeutic outcomes. Advancing pharmaceutical formulations and improving patient well-being depend on an awareness of the critical relevance of these “inactive” components.
Ultimately, the creation of drugs depends on excipients, which have a major impact on the effectiveness and approval of pharmaceutical goods. Their application will be enhanced by ongoing studies and regulatory oversight, hence ensuring more effective and safer pharmacological therapies for patients all over the world.
References
Handbook of Pharmaceutical Excipients by Raymond J Sheskey, 6th edition.

