Abstract
Recent advances in microbial bioengineering have redefined the landscape of mucosal immunization by enabling probiotic bacteria to serve as living drug delivery vehicles. This study introduces a dual-function, orally administered platform based on Escherichia coli Nissle 1917 (EcN) genetically engineered to deliver anti-SARS-CoV-2 nanobodies and spike antigens directly to the gastrointestinal tract. The system employs membrane-anchoring domains (Lpp-OmpA and Intimin) for surface display of nanobodies and leverages outer membrane vesicles (OMVs) as self-propelled nanocarriers to facilitate systemic and neuroimmune dissemination.
Upon oral administration, the engineered EcN not only elicited potent mucosal IgA and systemic IgG responses but also demonstrated pseudovirus neutralization capabilities in the gut, lungs, and brain tissues. Compared to conventional mRNA vaccines and monoclonal antibodies, the EcN-based platform demonstrated superior mucosal immune induction, self-renewing antigen presentation, and minimal immunotoxicity, thereby offering a highly modular, scalable, and needle-free alternative for global antiviral immunization. These findings underscore the potential of probiotic-based nanobody and OMV delivery systems as transformative tools in precision vaccinology and live biotherapeutic engineering. [1]
Introduction
Nalinikanth Kotagiri’s laboratory specializes in the bioengineering of probiotic bacteria to perform diverse therapeutic functions, ranging from dismantling cancer defense mechanisms to imaging and diagnosing pulmonary infections. Several years ago, the team investigated whether Escherichia coli Nissle 1917—a well-characterized probiotic strain—could be repurposed to deliver antiviral therapeutics or vaccine antigens directly to the gut, a primary site of viral entry. As a proof of concept, they focused on SARS-CoV-2, the causative agent of COVID-19. [1]
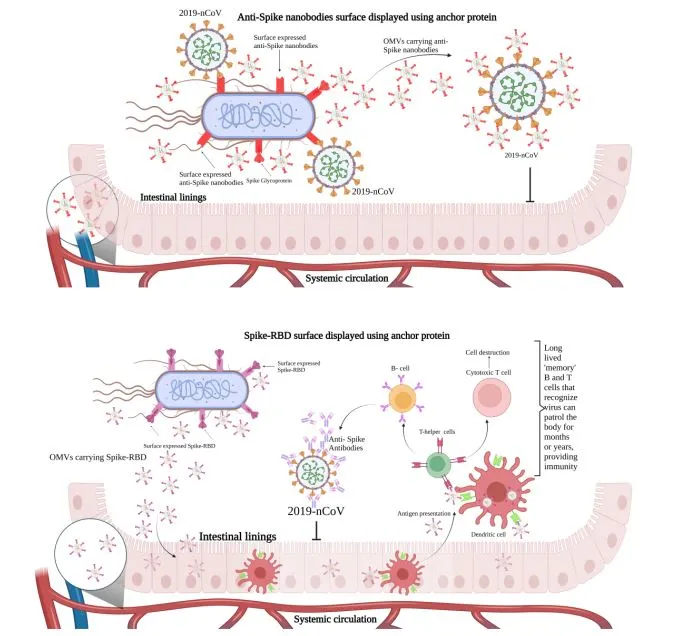
Unlike conventional engineered bacteria that retain therapeutic cargo intracellularly, effective vaccination requires antigen presentation to the immune system. To address this, the team engineered the probiotic to display viral proteins on its surface and leveraged outer-membrane vesicles (OMVs)—naturally secreted nanoscale vesicles—as self-propelled delivery vehicles. Upon release, OMVs traverse the gut epithelium, enter systemic circulation, and disseminate their payload to distal tissues. [1], [2]
Nitin S. Kamble, PhD, a research scientist in Kotagiri’s lab, conducted systematic screening of anchor motifs and expression cassettes to optimize surface antigen density. For the vaccine construct, the bacteria were programmed to express the SARS-CoV-2 spike protein—the same antigen utilized in mRNA vaccines. While current vaccines induce robust systemic immunity via circulating antibodies, mucosal surfaces—such as those in the gastrointestinal and respiratory tracts—remain vulnerable as primary viral entry points. Preclinical studies demonstrated that a two-dose oral regimen elicited systemic antibody titers comparable to intramuscular mRNA vaccination. Crucially, it also induced significantly elevated levels of secretory IgA (sIgA) in the gut and airways—the cornerstone of mucosal immunity, which is essential for preventing initial infection.
Beyond prophylactic vaccines, the team engineered a therapeutic variant of E. coli Nissle 1917 to express anti-spike nanobodies—compact, high-affinity antibody fragments—on its surface. Although full viral-challenge studies are pending, ex vivo assays confirmed that OMV-facilitated nanobodies reached the bloodstream and accumulated in lung tissue, effectively neutralizing SARS-CoV-2.
Unlike intravenous monoclonal antibodies, which require high doses, the probiotic serves as a self-renewing depot, sustaining therapeutic levels for extended periods. With the platform now optimized, Kotagiri envisions rapid adaptation for other pathogens, such as influenza and norovirus. Upcoming clinical trials will evaluate the safety and efficacy of this delivery system for other viral targets. To date, the engineered bacteria have exhibited an excellent safety profile in animal models, with no adverse immune reactions. Moreover, the parental strain has a long-established history of safe use as a probiotic. Looking ahead, Kotagiri proposes integrating both vaccine and therapeutic components within a single bacterial chassis. [1], [3]
Dr. Nalinikanth Kotagiri’s laboratory pioneers the bioengineering of Escherichia coli Nissle 1917, a probiotic strain, to function as an advanced oral delivery system for antiviral vaccines and therapeutics. Initially targeting SARS-CoV-2 as a proof of concept, the team engineered the bacteria to display spike proteins on their surface and secrete outer membrane vesicles (OMVs), which effectively traverse the gut barrier and deliver payloads systemically. This strategy enabled a dual immune response: systemic immunity comparable to mRNA vaccines and heightened mucosal immunity via elevated secretory IgA in the gut and respiratory tract—key to preventing viral entry. In parallel, they developed a therapeutic variant expressing anti-spike nanobodies, which reached lung tissue and neutralized the virus in ex vivo assays.
The system functions as a self-replicating therapeutic depot, contrasting with high-dose monoclonal antibody injections. With an excellent preclinical safety profile and scalable potential, the platform is now being optimized for pathogens beyond COVID-19, with future plans to integrate both vaccine and therapeutic functionalities into a single bacterial vector.
Design and Construction of Engineered EcN
A modular plasmid system was constructed to express nanobodies VHH72 and Ty1 on the bacterial surface using anchoring proteins (Intimin and Lpp-OmpA). The expression was validated by SDS-PAGE, Western blotting, and mass spectrometry. The system effectively produced surface-displayed nanobodies with confirmed binding affinity.
This research presents an innovative drug delivery system using Escherichia coli Nissle 1917 (EcN) as a probiotic platform to simultaneously provide passive and active immunization against viral pathogens, particularly SARS-CoV-2. EcN was genetically modified to display anti-spike nanobodies or spike receptor-binding domains (RBDs) on its surface. The platform demonstrated both mucosal and systemic immune responses upon oral administration, with the potential to be extended to other viruses. The rationale is rooted in the use of symbiotic bacteria to stimulate mucosal immunity.
Probiotic strains such as EcN can colonize the gut and interact with immune cells. Early systems focused on cytokine delivery for intestinal diseases, but advancements now target systemic antiviral applications. Oral delivery improves compliance, safety, and cost-effectiveness, bypassing the limitations of injection-based vaccines. [3]
In a sophisticated attempt to establish an orally deliverable nanobody-based antiviral platform, researchers designed a modular expression system tailored for Escherichia coli Nissle 1917 (EcN). This system was centered around the surface display of two potent nanobodies—VHH72 and Ty1—targeting the receptor-binding domain (RBD) of the SARS-CoV-2 spike protein. To ensure effective anchorage and presentation on the bacterial outer membrane, two well-characterized membrane-tethering motifs were employed: Intimin, a β-barrel transmembrane protein with an extended extracellular domain suitable for epitope presentation, and Lpp-OmpA, a hybrid construct known for stable and efficient outer membrane localization.
These constructs were integrated into plasmids driven by constitutive promoters to guarantee robust and continuous expression in vivo. The recombinant expression of the nanobodies was rigorously validated through SDS-PAGE and Western blotting, confirming size-appropriate bands, while tandem mass spectrometry and peptide mapping further substantiated correct folding and stable expression. Comparative analysis demonstrated successful display of nanobodies with high binding affinity, with the anchoring modality influencing the spatial orientation and, consequently, the bioavailability and functional efficacy of the nanobodies. These design elements underpin the ability of EcN to serve as both a passive neutralizer of viral particles and a dynamic carrier for immunogenic epitopes, setting the stage for dual-function antiviral mucosal therapeutics. [1], [3], [4]
Mechanism of Action via Nanobody Display
The engineered Escherichia coli Nissle 1917 (EcN) strains exert their antiviral effect through a precisely designed mechanism involving the surface display of nanobodies—small, single-domain antibody fragments derived from camelid heavy-chain antibodies—targeting the receptor-binding domain (RBD) of the SARS-CoV-2 spike glycoprotein. These nanobodies, namely VHH72 and Ty1, were genetically anchored onto the bacterial surface via two distinct membrane-tethering scaffolds: Lpp-OmpA and Intimin.
Once orally administered, these functionalized EcN strains colonize the gastrointestinal tract, where their surface-expressed nanobodies come into direct contact with spike protein-expressing viral particles or pseudoviruses within the intestinal lumen. In vitro fluorescence-based assays revealed that these nanobodies effectively blocked the interaction between spike RBD and human ACE2 receptors, thereby neutralizing viral entry pathways at mucosal surfaces. [1]
The architectural selection of anchoring proteins significantly influenced the orientation, stability, and inhibitory performance of the nanobodies. Lpp-OmpA, a hybrid lipoprotein construct, provided close and stable membrane tethering that allowed robust display in dense configurations, enhancing mechanical resistance and proteolytic protection. Conversely, Intimin, a large autotransporter protein, extended the nanobodies further into the extracellular space, enabling improved accessibility and binding to viral antigens but with potentially less structural rigidity.
Functional assays demonstrated that although both anchoring modalities achieved neutralization, constructs employing Lpp-OmpA-Ty1 displayed superior inhibition efficacy due to optimized spatial proximity and orientation for receptor engagement. This mechanistic insight underscores the importance of anchoring strategy as a critical design parameter in drug delivery system engineering, directly influencing the pharmacodynamic profile and antiviral potency of bioengineered probiotics in situ. [1], [5], [6]
Fluorescence-based assays confirmed this blockade of viral entry. Comparative analysis revealed that the Lpp-OmpA-Ty1 construct outperformed other configurations, achieving superior neutralization due to its dense, membrane-proximal presentation, enhancing nanobody stability, orientation, and receptor-binding efficiency. These findings highlight that the choice of anchoring scaffold is a pivotal determinant of therapeutic efficacy in mucosal-targeted bioengineered probiotics and profoundly impacts pharmacodynamic behavior and antiviral potency.
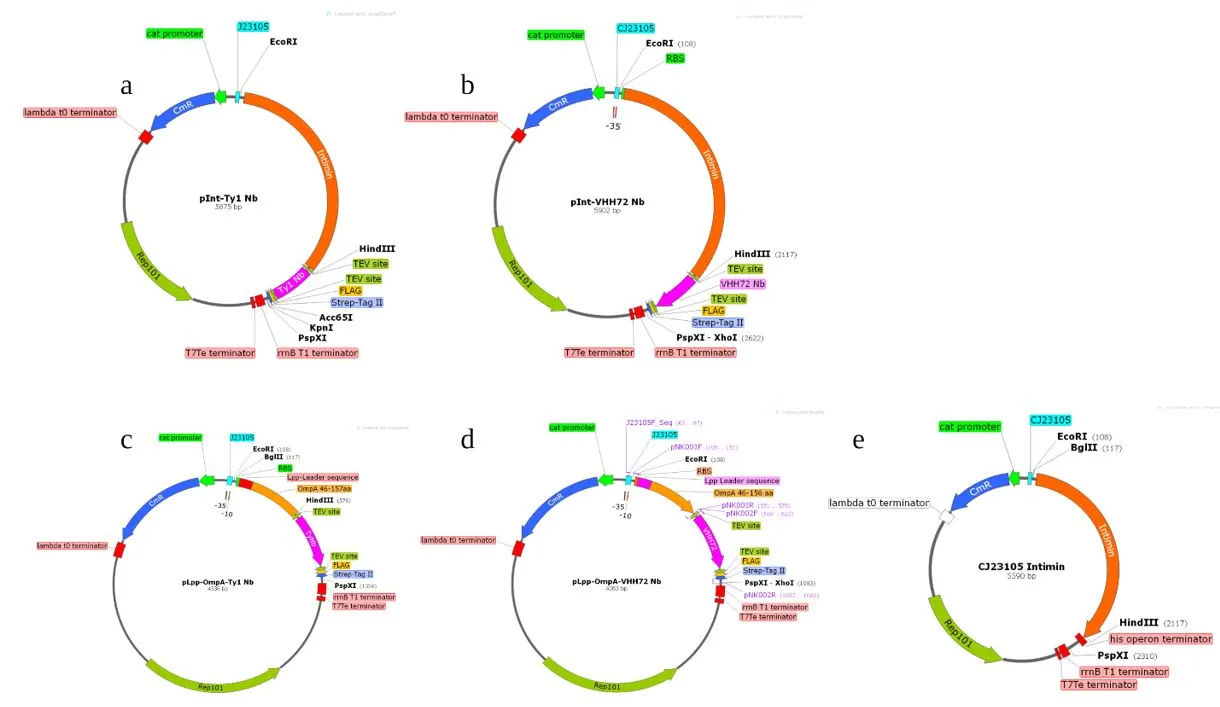
Plasmid constructs generated for displaying anti-COVID nanobodies on the cell surface [1]
Role of Outer Membrane Vesicles (OMVs)
Outer membrane vesicles (OMVs) derived from Escherichia coli Nissle 1917 (EcN) represent a pivotal advancement in microbial drug delivery engineering, acting as nanoscale carriers that encapsulate and transport bioactive payloads, such as antiviral nanobodies, across biological barriers. These OMVs, naturally secreted by Gram-negative bacteria, were isolated from engineered EcN strains expressing surface-tethered nanobodies (Ty1 and VHH72) and characterized using high-resolution transmission electron microscopy (TEM) and dynamic light scattering (DLS), confirming their spherical morphology and nanoscale size distribution (approximately 60–100 nm). These vesicles maintain structural integrity while encapsulating or displaying nanobodies on their surface, a critical feature enabling interaction with host tissues. [1]
The functional relevance of these OMVs lies in their ability to translocate nanobodies from the intestinal lumen into systemic circulation, subsequently distributing them to distant sites, such as pulmonary and neural tissues. This translocation was evidenced by immunohistochemical detection of nanobodies in lung and brain tissues of orally immunized mice, revealing that OMVs traversed both the gut-blood and blood-brain barriers—two formidable physiological hurdles in drug delivery. This was further supported by proteomic and immunoblotting analyses showing intact nanobody presence on OMVs, with anchoring strategies (e.g., Intimin vs. Lpp-OmpA) modulating vesicle surface architecture and loading efficiency. [1], [7]
The inherent immunogenicity, biocompatibility, and self-adjuvant properties of OMVs offer synergistic advantages for mucosal and systemic immunotherapies. By co-opting EcN’s colonization potential and vesiculogenic capacity, the platform amplifies antigen delivery and immune engagement while bypassing conventional parenteral administration routes. In essence, OMVs function as bioengineered exosomes, enabling targeted delivery of functional antibodies or antigens and expanding the therapeutic index of probiotic-based immunization strategies. Their utility thus spans beyond SARS-CoV-2 to potential applications against a spectrum of infectious and inflammatory diseases, affirming their role as a next-generation, multifunctional delivery system in drug delivery system engineering. [7]
Conclusion
This study marks a transformative advancement in drug delivery system engineering by developing a dual-functional, orally administered probiotic platform that integrates both passive and active immunization strategies. By leveraging the safe and well-characterized probiotic Escherichia coli Nissle 1917 (EcN), researchers engineered bacterial cells to express high-affinity nanobodies targeting the SARS-CoV-2 spike protein, while simultaneously anchoring spike antigens on their surface to stimulate adaptive immunity. The system exploits two major innovations: (1) the strategic surface display of therapeutic biomolecules via modular anchoring domains (Intimin and Lpp-OmpA), and (2) the utilization of outer membrane vesicles (OMVs) for targeted delivery beyond the intestinal mucosa.
This engineered construct, upon oral administration, effectively colonizes the gut, initiates localized immune responses at mucosal entry points, and facilitates systemic immune activation via nanobody-loaded OMVs. The capacity to neutralize viral particles and induce robust antigen-specific IgA and IgG responses, coupled with the ability of OMVs to traverse the gut-blood and even blood-brain barriers, underscores the systemic reach of this mucosal vaccine strategy. The prolonged bacterial persistence and the longitudinal elevation of mucosal and systemic IgA levels validate its potential as a sustained immunization platform.
Moreover, when benchmarked against conventional monoclonal antibodies and mRNA vaccines, the EcN-based delivery system exhibited comparable or superior performance in mucosal compartments, with additional benefits of non-invasive administration, enhanced patient compliance, and modularity for re-engineering against diverse pathogens. These findings establish this platform as a viable foundation for the development of customizable, next-generation live biotherapeutics and oral vaccines capable of addressing global immunization challenges with precision and safety.
This study marks a transformative advancement in drug delivery system engineering by developing a dual-functional, orally administered probiotic platform that integrates both passive and active immunization strategies. By leveraging the safe and well-characterized probiotic Escherichia coli Nissle 1917 (EcN), researchers engineered bacterial cells to express high-affinity nanobodies targeting the SARS-CoV-2 spike protein, while simultaneously anchoring spike antigens on their surface to stimulate adaptive immunity. The system exploits two major innovations: (1) the strategic surface display of therapeutic biomolecules via modular anchoring domains (Intimin and Lpp-OmpA), and (2) the utilization of outer membrane vesicles (OMVs) for targeted delivery beyond the intestinal mucosa.
This engineered construct, upon oral administration, effectively colonizes the gut, initiates localized immune responses at mucosal entry points, and facilitates systemic immune activation via nanobody-loaded OMVs. The capacity to neutralize viral particles and induce robust antigen-specific IgA and IgG responses, coupled with the ability of OMVs to traverse the gut-blood and even blood-brain barriers, underscores the systemic reach of this mucosal vaccine strategy. The prolonged bacterial persistence and the longitudinal elevation of mucosal and systemic IgA levels validate its potential as a sustained immunization platform.
Moreover, when benchmarked against conventional monoclonal antibodies and mRNA vaccines, the EcN-based delivery system exhibited comparable or superior performance in mucosal compartments, with additional benefits of non-invasive administration, enhanced patient compliance, and modularity for re-engineering against diverse pathogens. These findings establish this platform as a viable foundation for the development of customizable, next-generation live biotherapeutics and oral vaccines capable of addressing global immunization challenges with precision and safety.
Importantly, the engineering of anchoring motifs such as Lpp-OmpA and Intimin not only impacted antigen accessibility and nanobody stability but also allowed fine-tuning of therapeutic orientation, proteolytic resistance, and immunogenic profile, reinforcing the anchoring domain as a critical parameter in probiotic chassis design. The Lpp-OmpA–Ty1 configuration, in particular, demonstrated superior receptor blocking and neutralization activity, illustrating how subcellular localization engineering can decisively shape the therapeutic index of bioengineered probiotics. Additionally, the observed synergistic action between membrane-anchored nanobodies and OMV-mediated systemic delivery introduces a new paradigm in mucosal immunotherapy, where localized and systemic interventions are harmonized within a single microbial agent.
Looking forward, this adaptable platform opens avenues for rapid redeployment against emergent viral threats such as influenza, norovirus, or future coronaviruses, with potential expansion into bacterial and fungal pathogens. Integration of multiple immunogenic epitopes, programmable OMV cargo, or CRISPR-based biosensors could further enhance its versatility. Pending clinical validation, this system could revolutionize vaccine logistics by enabling refrigeration-free, orally self-administered biologics—a breakthrough for resource-limited settings and pandemic preparedness. Altogether, this work not only exemplifies a masterclass in synthetic probiotic design but also charts a clear translational path toward next-generation bioengineered mucosal vaccines and therapeutic delivery vectors in global health.