Introduction
Investigation of the Physico-chemical properties of the new drug compound that could affect drug performance and development of an efficacious dosage form is called a Preformulation study.
Preformulation is a group of studies that focus on the physicochemical properties of a new drug candidate that could affect the drug performance and the development of a dosage form.
Objectives
- Determining the physicochemical properties of a new medicinal entity
- Determining the kinetics and stability of that drug entity
- To see if it’s compatible with commonly used excipients.
- It reveals how drug items should be prepared and stored to maintain quality.
- It provides insights on how drug products should be processed & stored to ensure their quality
- To estimate problems that may arise during formulation, that as stability problems, poor in Vivo dissolution, and poor bioavailability
- To develop an optimal drug delivery system
Major area of preformulation study
| BULK CHARACTERS | SOLUBILITY ANALYSIS | STABILITY ANALYSIS |
| -Organoleptic properties -Crystallinity & Polymorphism -Hygroscopicity -Fine particle characterization -Powder flow properties | -Ionization constant-PKA -PH solubility profile -Common ion effect-Ksp -Thermal effects -Solubilization -Partition co-efficient -Dissolution | -Stability in toxicology formulations -Solution stability -pH rate profile -Solid-state stability -Bulk stability -Compatibility |
Bulk Characterization
1-Organoleptic properties
Color: It ought to be Unappealing to the attention and determined by either instrumental strategies or visible methodology that varies from batch to batch. Coating of the body with a variable color is done if found undesirable.
Odor and taste: Use a less soluble chemical version of the associated unappealing medication or mask it with flavors, excipients, coatings, Etc.
2-Crystallinity and Polymorphism
Crystalline Form:
A crystalline solid is a substance in which the atoms, ions, or molecules are organized in a highly structured and repeating three-dimensional configuration known as a crystal lattice. Crystalline solids exhibit a distinct and exact melting point because all the particles need the same quantity of energy to break free from the intermolecular forces that secure them in their lattice locations. Crystalline solids are categorized as “true solids.“
Key Characteristics:
- Long-range order
- Sharp melting point
- Definite shape
- Anisotropy
- Definite heat of fusion
Examples:
- Sodium chloride (NaCl) (Table Salt)
- Quartz (SiO₂)
- Diamond (C)
- Sugar (Sucrose, C₁₂H₂₂O₁₁)
Amorphous Form:
An amorphous solid is a substance in which the particles are arranged randomly and do not exhibit the long-range order typical of crystalline structures. Amorphous solids do not possess a distinct melting point; rather, they gradually begin to soften across a spectrum of temperatures. They usually display isotropic characteristics, indicating that their physical properties remain consistent in every direction. Amorphous solids are sometimes referred to as “pseudo-solids” or “supercooled liquids” due to their structural similarities to liquids.
Key Characteristics:
- Lack of long-range order:
- Melting over a range of temperatures
- Irregular shape
- Isotropy
- Irregular fracture
Examples:
- Glass (primarily SiO₂ with other additives)
- Rubber (natural and synthetic)
- Plastics (e.g., Polyethylene, Polystyrene)
- Wax
- Gels
Amorphous Solid vs Crystalline Solid
| Property | Amorphous Solid | Crystalline Solid |
| Structure | Lacks a well-defined, ordered structure & randomly distributed. | It has a sharp, distinct melting point. |
| Melting Point | Tend to soften over a range of temperatures. | Have greater solubility than their crystalline forms. |
| Transparency | Often translucent or opaque. | Can be transparent or translucent. |
| Stability | Have greater solubility than their crystalline forms. | These forms are more stable than amorphous forms. |
| Solubility | Have greater solubility than its crystalline forms. | Less solubility than their amorphous form. |
| Anisotropy | Typically isotropic, with uniform properties. | Anisotropic properties vary with direction. |
| Examples | Rubber, glass, plastic. | Diamond, salt, quartz. |
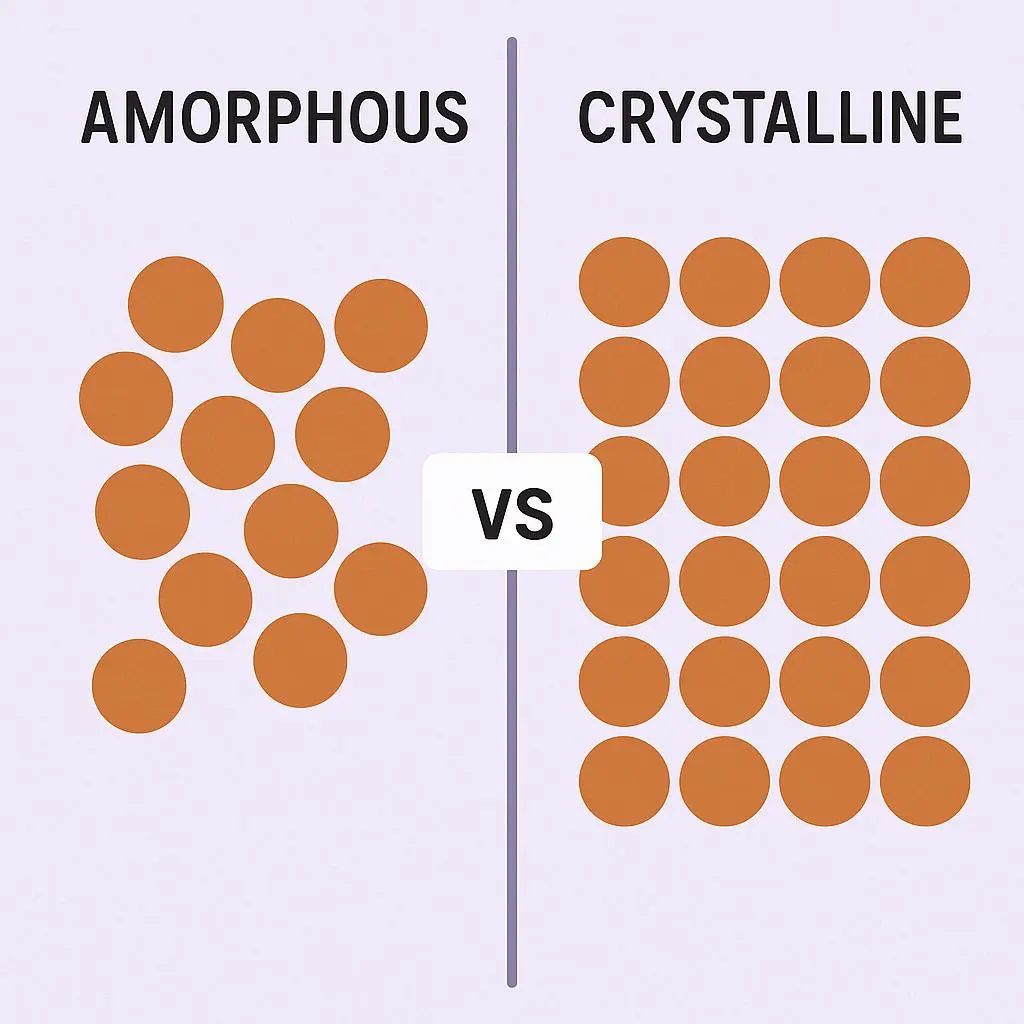
Figure 1: Molecular arrangement of a Crystalline solid and an Amorphous solid
Polymorphism
The ability of a solid material (a drug substance or an excipient) to exist in two or more crystalline forms that have different arrangements or conformations of the molecules in the crystal lattice is called polymorphism.
These different crystalline forms are called polymorphs. Although they have the same chemical formula, polymorphs can exhibit different physicochemical properties, such as:
- Melting point
- Solubility
- Dissolution rate
- Stability (both physical and chemical)
- Hygroscopicity (tendency to absorb moisture)
- Flowability
- Compactability
Types of Polymorphism:
Polymorphism can be broadly classified based on the thermodynamic relationship between the different forms:
Monotropic Polymorphism: A polymorph that is unstable at all temperatures. & pressure is called Monotropic polymorph. This transition is irreversible.
Enantiotropic Polymorphism: An enantiotropic polymorph is a type of polymorph that can switch between different forms when temperature or pressure is altered. There exists a transition temperature at which both forms possess identical free energy, allowing them to transform back and forth through heating or cooling.
Pseudo polymorphism (Solvates and Hydrates): These are crystal forms where solvent molecules (in solvates) or water molecules (in hydrates) are incorporated into the crystal lattice.
Solvates VS Hydrates:
These molecular complexes entrap the mother liquor molecules into specific sites within the crystal lattice and have a stoichiometric number of solvent molecules complexed. If the incorporated solvent is water, then the complex is called hydrate, while if the solvent is other than an aqueous system, then the complex is defined as solvates.
Depending on the ratio of water molecules within a complex, these can be categorized as follows:
- Anhydrous : 1 mole compound + 0 mole water
- Semi-hydrate: 1 mole compound + ½ mole water
- Monohydrate: 1 mole compound + 1 mole water
- Dihydrate : 1 mole compound + 2 moles water
Example: Carbamazepine
Carbamazepine, an anticonvulsant drug, is a well-known example of a polymorphic drug. It exists in several polymorphic forms, with different properties:
- Form I: This was the initially marketed form.
- Form III: This form is generally more soluble than Form I, potentially leading to better bioavailability.
- Dihydrate Form: Carbamazepine can also exist as a dihydrate, a pseudo-polymorph containing two water molecules per molecule of carbamazepine. The dihydrate typically has lower solubility compared to the anhydrous form.
These distinct carbamazepine polymorphs have important ramifications for the medication’s stability, formulation, processing, and, eventually, clinical effectiveness. A formulation that uses a less soluble form, for example, may have sub-therapeutic effects due to low dissolution and absorption. On the other hand, during storage, a more soluble but less stable form may change into a less soluble form, which could impact the product’s performance and shelf life.
Effect of polymorphism on dissolution and bioavailability:
Dissolution Rate
Solubility Differences:
Polymorphs display different levels of thermodynamic stability. Typically, metastable polymorphs (the less stable varieties) possess greater solubility in water than their more stable counterparts. This enhanced solubility may result in a quicker dissolution rate.
Crystal Lattice Energy:
The energy required to break the crystal lattice during dissolution differs among polymorphs. Metastable forms with higher energy states require less energy for dissolution, resulting in quicker release of the drug molecules into the surrounding fluid.
Surface Area and Morphology:
Different polymorphs can have distinct crystal shapes and surface areas. A larger surface area allows for greater interaction with the dissolution medium, enhancing the dissolution rate.
Bioavailability
Absorption Rate and Extent:
As dissolution frequently serves as the limiting factor for the oral absorption of poorly soluble medications, the rate of dissolution has a direct effect on bioavailability. Increasing the speed and completeness of dissolution results in improved absorption and greater bioavailability, which indicates that a larger portion of the delivered drug enters the systemic circulation.
Therapeutic Efficacy:
Variations in bioavailability caused by polymorphism can influence the effectiveness of a drug. A polymorph that is not easily soluble and dissolves slowly could result in inadequate therapeutic levels, whereas a more soluble variant might lead to a quicker onset of action and enhanced clinical results..
Example: Ritonavir
A classic example illustrating the impact of polymorphism on dissolution and bioavailability is the case of Ritonavir, an antiviral drug.
Form I: The initially marketed form of Ritonavir was less soluble and exhibited poor bioavailability, requiring high doses.
Form II: A more stable and less soluble polymorph (Form II) appeared during manufacturing, leading to significantly reduced dissolution rates and bioavailability. This necessitated a reformulation of the drug product
.Amorphous Form: Amorphous form of Ritonavir shows significantly higher solubility and bioavailability compared to both crystalline forms, allowing for a lower dose and improved therapeutic outcomes.
3-Hygroscopicity
The ability of a substance to absorb moisture (water vapor) from the surrounding atmosphere is called Hygroscopicity. This term occurs because the substance has a chemical or physical affinity for water molecules, leading to their adsorption or absorption onto or into the material.
Classification:
Hygroscopicity is classified into various types based on the extent of moisture uptake under specific conditions of temperature and relative humidity (RH).
On the basis of the European Pharmacopoeia (Ph. Eur..), materials after being stored at 25°C and 80% RH for 24 hours:
- Non-hygroscopic: Increase in mass is less than or equal to 0.2% w/w.
- Slightly hygroscopic: Increase in mass is greater than 0.2% w/w and less than 2.0% w/w.
- Hygroscopic: Increase in mass is greater than or equal to 2.0% w/w and less than 15.0% w/w.
- Very hygroscopic: Increase in mass is greater than 15.0% w/w.
Another classification system by Callahan et al. categorizes hygroscopicity based on the percentage weight gain after one week at different relative humidity levels:
- Class I: Non-Hygroscopic: At relative humidity below 90%, there are almost no moisture increases. Furthermore, after a week of storage at above 90% relative humidity (RH), the rise in moisture content is less than 20%.
- Class II: Mildly Hygroscopic: At relative humidity below 80%, virtually little moisture increases. The increase in moisture content after a week of storage at above 80% RH is less than 40%.
- Class III: Moderately Hygroscopic: At relative humidity below 60%, moisture content does not grow more than 5% after storage. The rise in moisture content after a week of storage at above 80% RH is less than 50%.
- Class IV: Very Hygroscopic (Deliquescent): At relative humidity as low as 40–50 percent, moisture might increase. After a period of storage, the moisture content of the product increases. Absorbs enough moisture to form a liquid solution.
Effect of Hygroscopicity on Product Quality:
Hygroscopicity can significantly impact the quality of pharmaceutical products in various ways:
Physical Stability:
- Caking and Clumping: Moisture absorption can lead to the formation of solid bridges between particles, causing powders and granules to cake or clump. This affects flowability, making processing (e.g., tablet manufacturing, capsule filling) difficult and can lead to weight variations in dosage units.
- Changes in Solid State: Hygroscopic materials can change their crystalline form (polymorphic transitions), form hydrates (if water is incorporated into the crystal lattice), or even become amorphous. These changes can alter solubility, dissolution rate, and stability.
Chemical Stability:
- Hydrolysis: Absorbed moisture can act as a reactant or a catalyst for hydrolytic degradation of drugs and excipients, leading to the formation of impurities and a reduction in the active drug content, thus affecting the efficacy and safety of the product.
- Microbial Contamination: Increased moisture content can create a favorable environment for the growth of microorganisms, leading to product spoilage and potential health hazards.
- Dissolution and Bioavailability: Changes in the solid state or the formation of hydrates due to hygroscopicity can alter the drug’s solubility and dissolution rate, which are critical factors for its absorption and bioavailability.
4-Characterization of fine particles:
Particle Size
Particle size is characterized using these terms :
- i. Very coarse (#8)
- Coarse (#20)
- iii. Moderately coarse (#40)
- iv. Fine (#60)
- v. Very fine (#80)
Particle size can influence variety of important factors :
- Dissolution rate
- Suspend ability
- Uniform distribution
- Penetrability
- Lack of grittiness
Fine particle characterization can be done by various methods:
- Microscopy
- Microscopy on a hot stage
- Thermo graphic analysis
- Diffraction of X-rays
- Infrared spectroscopy (IR)
- Proton magnetic resonance (PMR) is a technique for detecting protons
- Nuclear Magnetic Resonance (NMR) (NMR)
- Scanning electron microscopy (SEM) is the eighth technique (SEM)
5-The properties of powder flow
Changes in particle size, density, shape, and adsorbed moisture, which might occur during processing or formulation, have a substantial impact on flow characteristics. The flow properties depends upon following:
- Force of Friction
- Cohesion between one particle to another.
Bulk Density: It is calculated when solid is non porous i.e. true & granular density are identical so measured by Mercury displacement or Helium densitometer. It is ratio of bulk weight to bulk volume Bulkiness is reciprocal of bulk density. Bulk Density = Weight of the powder/ Bulk Volume
Tapped Density: Weight is taken and poured into a graduated cylinder. The cylinder is tapped 1000 times on a mechanical tapper apparatus. The volume reached a minimum – called tapped volume.
Tapped Density = Weight of the powder/ Tapped
The following approaches can be used to determine the powder flow properties:
The Angle of Repose:
It is the maximum angle formed by a pile of powder’s surface and the horizontal plane. {tan θ= h/r}
The angle of repose will be larger if the particle surface is rougher and more irregular. The lower the value, the better the flow properties.
The following are the acceptance criteria for angle of repose:-
| Angle of Repose | Type of Flow |
| < 20 | Excellent flow |
| 20-30 | Good flow |
| 30-34 | Passable |
| >40 | Poor flow |
The Carr’s index: A Car’s Index, also known as Carr’s Compressibility Index, is a measure of the powder’s compressibility and an indicator of its flowability. It is calculated from the bulk density and tapped density of the powder:
Carr’s index (%) = (Tapped density -bulk density x 100) / Tapped density
It is a simple, fast & popular method of predicting powder flow characteristics. Good flow characteristics can be attained by lowering the bulk and tapped density.
The following are the approval criteria for Carr’s index:
| % of Compressibility | Relative Flowability |
| 5-15 | Excellent |
| 12-16 | Good |
| 18-21 | Fair to passable |
| 23-35 | Poor |
| 33-38 | Very poor |
| > 40 | Extremely poo |
Hausner’s ratio: The ratio of the tapped density to the bulk density is called Hausner’s ratio.
Hausner’s ratio: = Tapped density / Bulk density
The following are the approval criteria for Hausner’s ratio:
| Hausner’s ratio: | Type of flow |
| 1.00–1.11 | Excellent |
| 1.12–1.18 | Good |
| 1.19–1.25 | Fair |
| 1.26–1.34 | Passable |
| 1.35–1.45 | Poor |
| 1.46–1.59 | Very poor |
References
-Komal Bhusare, et al. (2024). Preformulation Studies An Overview. INTERNATIONAL JOURNAL OF PHARMACEUTICAL SCIENCES.
-MANGESH G. BHISE, et al. (2014). A REVIEW ON PREFORMULATION STUDIES. Journal of emerging Technologies and Innovative Research.
-Priyanka Maurya, et al. (2016). Pharmaceutical polymorphism: The phenomenon affecting the performance of drug. World Journal of Pharmaceutical Sciences.

