Overview of Pharmaceutical Granulation
Pharmaceutical granulation is one of the main processes in the pharmaceutical manufacturing of almost all solid dosage forms, whether tablets, capsules, caplets, sachets, etc.
Solid-dosage forms encompass the largest category of dosage forms that are clinically used. Several types of tablet solid dosage forms are designed to optimize the absorption rate of the drug, increase the ease of administration by the patient, control the rate and site of drug absorption, and mask the taste of a therapeutic agent. This also applies to the capsules of various sizes and various release profiles.
The formulation of tablets and capsules involves the use of several components, each of which is present to facilitate the manufacture or to control the biological performance of the dosage form.
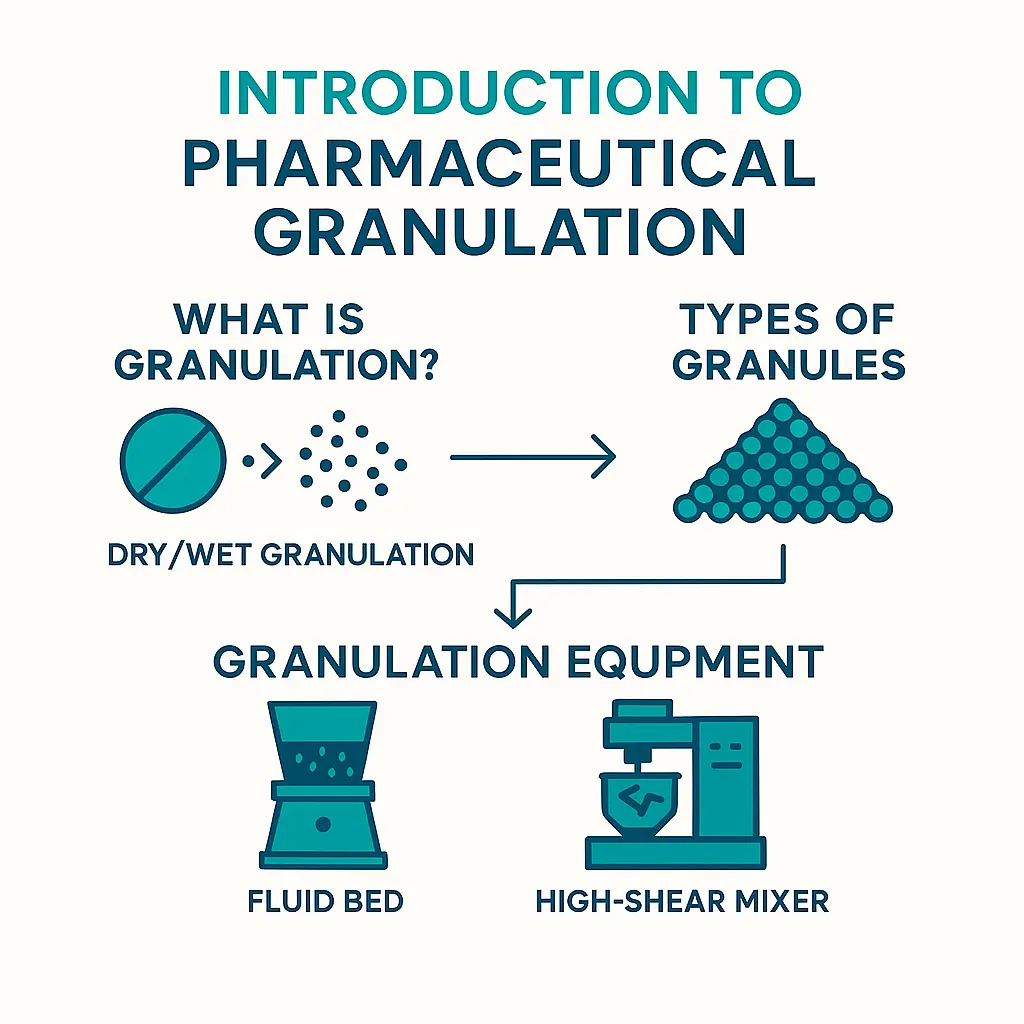
Granulation is a critical process in pharmaceutical manufacturing that involves the agglomeration of fine powder particles into larger, more uniform granules. This process improves the flow properties, compressibility, and uniformity of powders, making them suitable for tablet compression or capsule filling.
Perry’s Chemical Engineer’s Handbook defines the granulation process as “any process whereby small particles are gathered into larger, permanent masses in which the original particles can still be identified.”
This definition is appropriate to a pharmaceutical granulation where the rapid breakdown of agglomerates is important to maximize the available surface area and aid in the dissolution of the active drug. The granulated material can be obtained by direct size enlargement of primary particles or size reduction from dry, compacted material.
The final quality attributes of the dosage form will be based on the process of technology to be employed. The selection of the process also entails ancillary equipment that could have an impact on the granule properties; hence, characterizing the granulation for its flowability, morphological properties, and impact on bioavailability is necessary.
Importance of Granulation
- Improved Flowability: Due to the nature of fine powders, especially drug substances that often have poor flow properties, leading to inconsistent filling in dies during tablet compression. Granulation enhances flow characteristics, ensuring that uniform weight and content uniformity are accurately attained.
- Enhanced Compressibility: Granules have optimized compaction properties compared to raw powders, leading to stronger oral solid dosage forms with a lower probability of capping and lamination tendencies.
- Uniform Distribution of Active Pharmaceutical Ingredients (APIs): Granulation ensures homogeneous mixing of APIs and excipients, minimizing segregation.
- Dust Reduction: Granulation reduces dust generation, serving both operator safety and minimizing cross-contamination.
- Controlled Release: Modified-release formulations often require granulation to achieve desired drug release profiles.
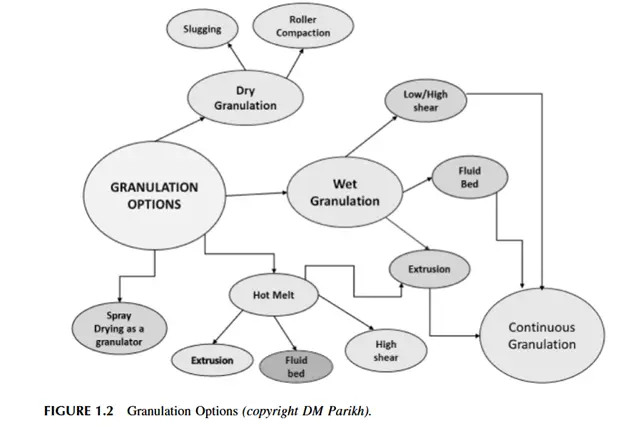
Types of Granulation Techniques
Granulation techniques are classified into two main categories:
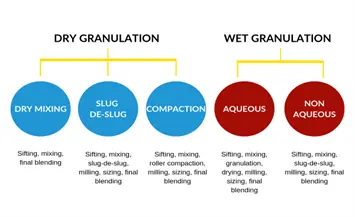
Wet Granulation
Wet granulation is the most widely used method, involving the addition of a liquid binder (water-based or solvent-based; either with alcohol or isopropanol) to the powder blend to form agglomerates.
Steps in Wet Granulation
- Mixing: Dry powders (API + excipients) are blended uniformly.
- Binder Addition: A liquid binder (e.g., povidone, hypromellose, starch paste) is added to form a wet mass.
- Wet Massing: The mixture is kneaded to form a cohesive mass.
- Granulation: The wet mass is passed through a sieve to form granules.
- Drying: The granules are dried using fluidized bed dryers or oven tray dryers to attain optimum moisture content.
- Sizing: Dried granules are milled and sieved to achieve uniform particle size.
- Lubrication: A lubricant (e.g., magnesium stearate) is added before compression.
Advantages of Wet Granulation
- Produces dense, strong granules.
- Improves content uniformity.
- Suitable for moisture-sensitive drugs (if non-aqueous binders are used).
Disadvantages of Wet Granulation
- Requires multiple processing steps.
- Not suitable for heat- or moisture-sensitive drugs (unless optimized).
- Longer processing time compared to dry granulation.
Dry Granulation
Dry granulation is used when the drug substance or inactive components are sensitive to moisture or heat. This method involves compacting powders without using a liquid binder.
Methods of Dry Granulation
- Slugging: This method is normally used in lab-scale preparations. Bulk powders are compressed into large tablets (slugs), which are then milled and sieved into granules.
- Roller Compaction: Used in large-scale production where bulk powders are compressed between rollers to form ribbons or sheets, which are then milled into granules.
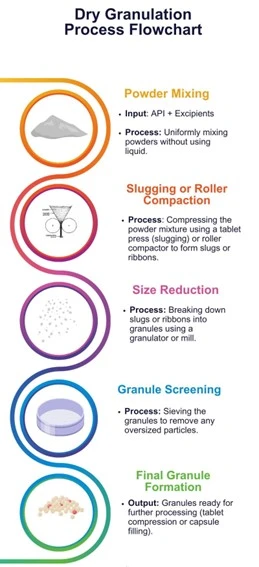
Advantages of Dry Granulation
- No moisture or heat is involved, suitable for moisture and heat-sensitive drugs.
- Faster process compared to wet granulation.
- Lower equipment and operational costs.
Disadvantages of Dry Granulation
- Produces less dense granules.
- Higher risk of segregation.
- Needs reprocessing if not reached the optimum percentage of coarse granules.
Other Pharmaceutical Granulation Techniques
- Melt Granulation: Uses meltable binders (e.g., polyethylene glycol, waxes) instead of liquid binders to enhance low melting point APIs granulation.
- Fluidized Bed Granulation: Combines mixing, granulation, and drying in a single equipment in large-scale production.
- Spray Drying Granulation: Converts liquid formulations into dry granules via atomization and drying.
- High Shear Granulation: Uses high-speed mixing to produce dense granules.
Pharmaceutical Granulation Equipment
Various granulation equipment exists in the pharmaceutical field, as demonstrated as follows:
Wet Granulation Equipment
- High-Shear Mixer Granulators: Use mechanical agitation assisted by shear forces for granule formation.
- Fluidized Bed Granulators: Industrial-scale equipment that combines granulation and drying in a single machine.
- Planetary Mixers: Used for small-scale granulation.
Dry Granulation Equipment
- Roller Compactors: These work by compacting powders into ribbons or sheets before milling.
- Tablet Presses (for Slugging): Used to form slugs before granulation, as explained above.
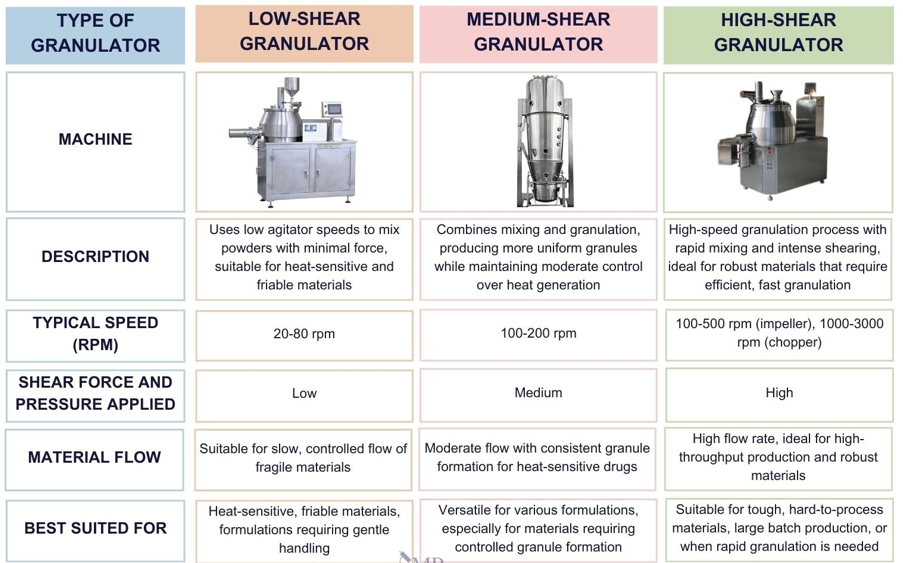
Critical Process Parameters in Pharmaceutical Granulation
Several factors influence granule quality; these items will be discussed in detail in our next articles, but currently, we will briefly state some of them:
- Binder Selection & Concentration: Affects granule strength and dissolution.
- Granulation Time & Shear Forces: Over-granulation can lead to hard granules, while under-granulation results in weak granules.
- Drying Temperature & Time: Excessive heat can affect heat-sensitive APIs.
- Particle Size Distribution: Affects blend, content, tablet uniformity, and dissolution.
Quality Control in Pharmaceutical Granulation
Granules must meet specific quality standards:
- Particle Size Distribution (Sieve analysis)
- Moisture Content (Loss on drying, Karl Fischer device)
- Flow Properties (Angle of repose, Carr’s index, Hausner ratio)
- Compressibility & Tabletability
Recent Advances in Pharmaceutical Granulation Technology
- Continuous Granulation: Improves efficiency and reduces batch-to-batch variability.
- Process Analytical Technology (PAT): Real-time monitoring of granulation using NIR, Raman spectroscopy.
- 3D Printing Granulation: Customized granule production for personalized medicine.
Conclusion
Granulation is a fundamental process in pharmaceutical manufacturing, ensuring the production of high-quality solid dosage forms. The various discussed techniques will further help the scientists in their understanding and selection of the granulation process most appropriate for the drug in question.
By understanding the principles, techniques, and critical parameters of granulation, pharmaceutical scientists can optimize formulations for better therapeutic outcomes.


