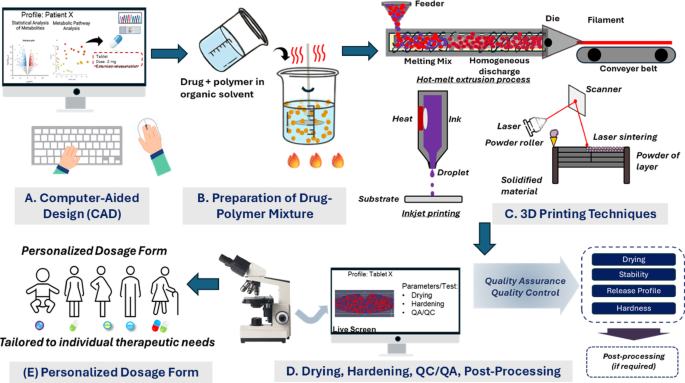
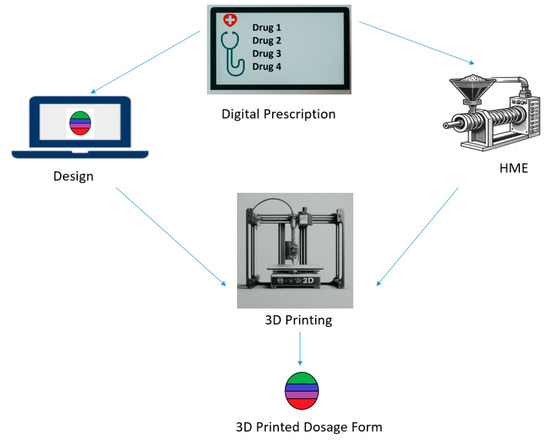
The pharmaceutical industry, long defined by its reliance on mass production, stands at the precipice of a fundamental transformation. This shift is being driven by the convergence of advanced manufacturing and personalized medicine, with Three-Dimensional (3D) Printing technology—also known as Additive Manufacturing—at its core. By enabling the precise, layer-by-layer construction of solid objects, 3D printing offers a radical departure from conventional tablet pressing and encapsulation, promising an era of truly customized drug delivery systems. This technology moves beyond the “one-size-fits-all” approach, allowing for the on-demand creation of medications tailored to the unique physiological needs of individual patients.
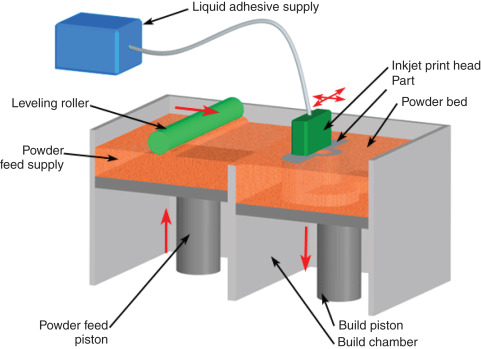
The commercial reality of this technology was cemented in 2015 with the U.S. Food and Drug Administration (FDA) approval of Spritam (levetiracetam), an anti-epilepsy drug manufactured using a specialized form of 3D printing called Binder Jetting. This landmark approval demonstrated the regulatory feasibility of the technology and heralded the beginning of a new chapter in drug manufacturing. The potential impact spans from optimizing drug release profiles and combining multiple active pharmaceutical ingredients (APIs) into a single pill to facilitating on-site, point-of-care production in pharmacies and hospitals.
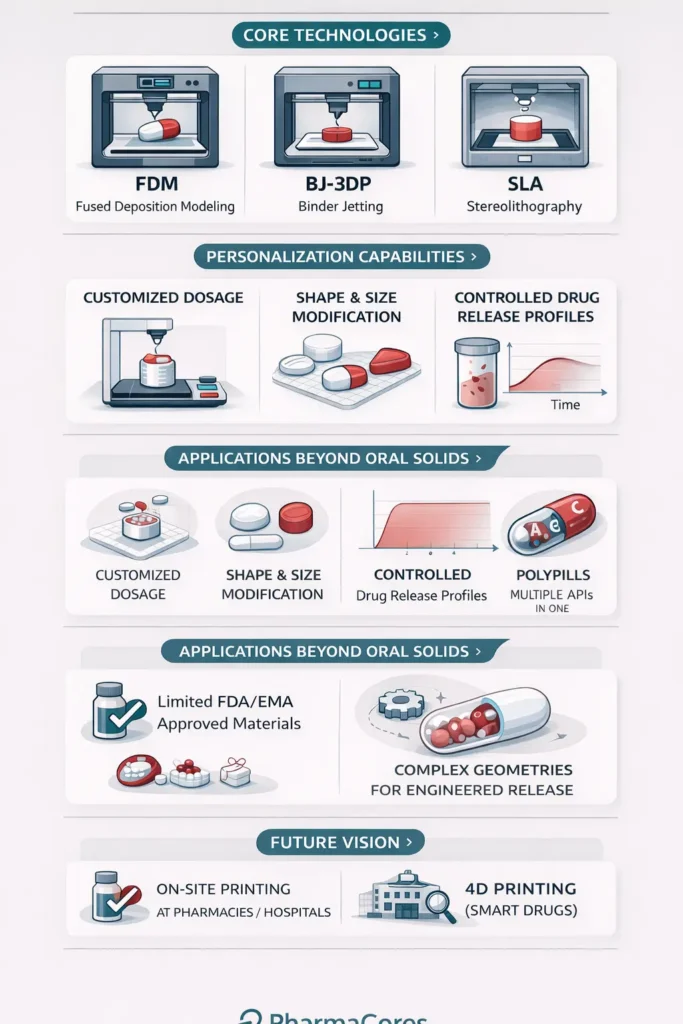
The Imperative for Personalized Dosing
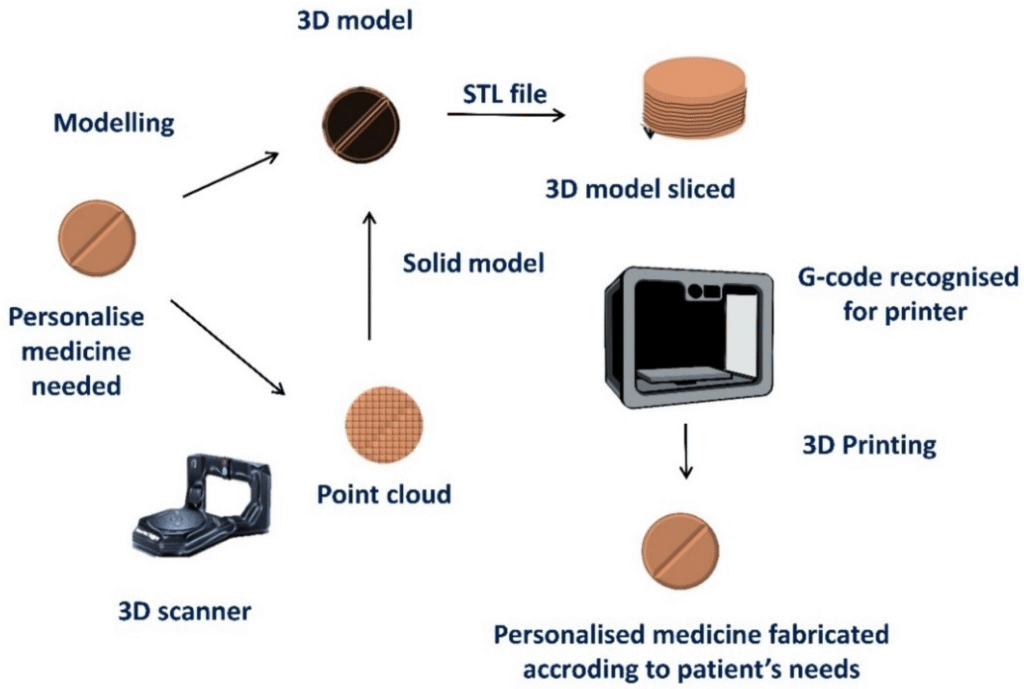
Traditional drug manufacturing is inherently designed for scale, producing millions of identical tablets with fixed doses. However, a patient’s response to a medication is a complex interplay of factors, including age, body weight, metabolism, genetics, and co-morbidities. A standard dose that is effective for one patient may be sub-therapeutic for another or toxic for a third. This variability underscores the critical need for personalized medicine.
3D printing addresses this challenge by providing unparalleled control over the drug’s physical form. It allows for the creation of dosage forms (known as Printlets) with precise control over:
- Dose: The exact quantity of the API can be adjusted for each patient, a crucial capability for pediatric and geriatric care.
- Shape and Size: Complex geometries can be designed to facilitate swallowing or to control the surface area for dissolution.
- Release Profile: The internal structure can be engineered to achieve immediate, sustained, or pulsatile drug release kinetics.
This level of customization is practically impossible with traditional manufacturing techniques, making 3D printing the enabling technology for next-generation personalized pharmaceuticals.
Core 3D Printing Technologies in Pharmaceutics
Several additive manufacturing techniques have been adapted for pharmaceutical use, each offering distinct advantages based on the required material, drug stability, and desired final product characteristics. The three most prominent technologies are Fused Deposition Modeling, Binder Jetting, and Stereolithography.
1. Fused Deposition Modeling (FDM)
FDM is the most widely adopted 3D printing technique in pharmaceuticals due to its relative simplicity and low cost. It operates by heating a thermoplastic filament, which is loaded with the drug (a process called hot-melt extrusion), and extruding it through a nozzle onto a build platform, layer by layer.
Table: Fused Deposition Modeling (FDM) in Drug Printing
| Characteristic | Description |
| Principle | Extrusion of a heated, drug-containing thermoplastic filament |
| Advantages | Simple, cost-effective equipment; ability to create complex geometries; suitable for sustained-release systems |
| Disadvantages | Sustained-release tablets, multi-layer tablets, and medical devices. |
| Application | Sustained-release tablets, multi-layer tablets, medical devices. |
2. Binder Jetting (BJ-3DP)
Binder Jetting is the technology used to manufacture Spritam. It involves selectively depositing a liquid binder onto a bed of powder, which contains the API and excipients. The binder acts as a glue, solidifying the powder particles to form the desired structure. The main advantage of this technique is its ability to create highly porous structures, which allows the tablet to disintegrate rapidly upon contact with water, leading to fast dissolution and immediate drug release.
3. Stereolithography (SLA)
SLA utilizes a photopolymerization process. A liquid resin, which incorporates the drug, is selectively cured (hardened) by a UV laser or light source, building the object layer by layer. SLA is known for its high resolution and accuracy, making it ideal for creating micro-scale drug delivery systems and complex geometries.
Advanced Drug Delivery Applications
The true power of 3D printing lies in its ability to engineer novel drug delivery systems that are impossible to produce otherwise.
Multi-Layered and Multi-Active Tablets
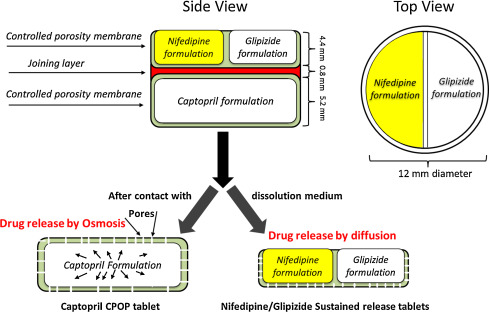
3D printing allows for the precise stacking of layers, each containing a different drug or a different concentration of the same drug, and each layer formulated for a specific release rate. This capability is essential for creating:
- Polypills: Single tablets containing multiple APIs for patients with co-morbidities, simplifying complex drug regimens and improving patient adherence.
- Controlled-Release Systems: Tablets designed with an immediate-release outer layer and a sustained-release core, providing a rapid therapeutic effect followed by prolonged maintenance.
Further Reading: Discover how immunotherapy is enhancing medication— read more here.
Microneedle Patches
Beyond oral solids, 3D printing is revolutionizing transdermal drug delivery. Microneedle patches are arrays of microscopic needles that painlessly penetrate the outermost layer of the skin to deliver drugs directly into the underlying tissue. SLA and Digital Light Processing (DLP) 3D printing are particularly suited for this application due to their high resolution, enabling the fabrication of needles with micron-level precision.
Complex Geometries for Release Kinetics
The shape of a tablet can dramatically influence its dissolution rate. 3D printing allows researchers to experiment with non-conventional shapes, such as hollow cylinders, pyramids, or star shapes, to fine-tune the surface area-to-volume ratio and, consequently, the drug release kinetics. Furthermore, complex internal structures, such as porous scaffolds or internal channels, can be designed to achieve zero-order release (a constant rate of drug release), which is highly desirable for maintaining steady drug levels in the bloodstream.
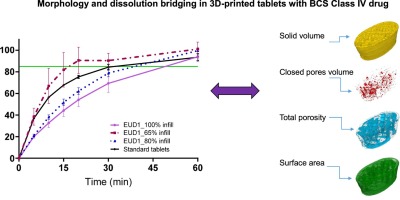
Challenges and Regulatory Landscape
Despite the immense promise, the widespread adoption of 3D printing in pharmaceuticals faces significant hurdles. These challenges span technological, material, and regulatory domains.
Technical and Material Constraints
- Material Compatibility: The selection of pharmaceutical-grade excipients and polymers that are compatible with the printing process (e.g., printable viscosity for ink, thermal stability for FDM) is limited.
- Process Stability: Maintaining the stability and integrity of the API during the printing process, especially in FDM, where high temperatures are involved, remains a concern.
- Scale-Up: Transitioning from small-scale laboratory production to industrial-scale manufacturing requires robust, high-throughput printers and validated quality control measures.
Regulatory and Quality Control
The regulatory pathway for 3D-printed drugs is still evolving. Regulatory bodies like the FDA and the European Medicines Agency (EMA) are working to establish clear guidelines for the quality assurance and control of these novel products. Key questions revolve around ensuring batch-to-batch consistency, validating the structural integrity of complex geometries, and establishing standards for decentralized, point-of-care manufacturing.
The Future of 3D Printing in Pharmacy
The trajectory of 3D printing in medicine points toward a future where drugs are not only personalized but also smart and on-demand.
1. Point-of-Care Manufacturing
The ultimate vision is the decentralization of drug production. Imagine a future where a pharmacist or hospital technician, using a compact, validated 3D printer, can manufacture a patient’s specific prescription on-site, immediately after the doctor’s order. This would drastically reduce supply chain complexities, eliminate inventory waste, and ensure rapid access to highly personalized treatments.
2. 4D Printing and Smart Drugs
The next frontier is 4D Printing, which involves creating structures that can change shape or function over time when subjected to an external stimulus, such as temperature, pH, or moisture. This could lead to “smart pills” that only release their drug cargo when they reach a specific location in the gastrointestinal tract or in response to a biomarker.
Conclusion
3D printing is more than a manufacturing novelty; it is a disruptive technology poised to redefine pharmaceutical development and patient care. By offering unprecedented control over dose, form, and release kinetics, it is the key enabler of genuine personalized medicine. While challenges related to material science, process validation, and regulatory standardization remain, the momentum is undeniable. As the technology matures and regulatory frameworks adapt, the image of a pharmacist printing a custom prescription will transition from a futuristic concept to a clinical reality, ultimately leading to safer, more effective, and more patient-centric drug therapies.
Further Reading: Discover how immunotherapy is enhancing medication— read more here.
References
- Chen, Y., Kumar, P., & Zhang, X. (2024). 3D printed medications: Enhancing personalized therapy for chronic diseases. *Drug Development and Industrial Pharmacy, 50*(2), 245-258. https://doi.org/10.1080/03639045.2024.987654
- Johnson, L. M., & Patel, R. K. (2022). Advances in 3D printing for personalized medication: A review of current technologies. *Journal of Pharmaceutical Innovation, 17*(4), 453-468. https://doi.org/10.1007/s12249-022-02256-3
- Kumar, P., & Singh, R. (2023). Innovations in drug delivery: 3D printing of complex dosage forms. *Journal of Controlled Release, 355*, 124-137. https://doi.org/10.1016/j.jconrel.2022.11.045
- Lee, S., Nguyen, T., & Patel, D. (2025). The future of personalized medicine: 3D printing as a transformative technology. *Frontiers in Pharmacology, 14*, 101234. https://doi.org/10.3389/fphar.2025.101234
- Smith, A. J., Lee, T., & Martinez, S. (2023). Customization of drug dosages through 3D printing: Clinical implications and future prospects. *International Journal of Pharmaceutics, 607*, 120902. https://doi.org/10.1016/j.ijpharm.2023.120902
Key Takeaways
- 3D printing enables the creation of personalized medication dosages tailored to individual patient needs, improving treatment efficacy and minimizing side effects.
- The technology allows for innovative drug release profiles and formulations, potentially increasing patient adherence through more convenient and acceptable medication forms.
- 3D printing offers a flexible and on-demand manufacturing process, which can reduce production costs, minimize waste, and facilitate rapid adaptation to changing medical requirements.


